Oxidative destruction of anionite AV-17×8 using the Fenton reaction
Abstract
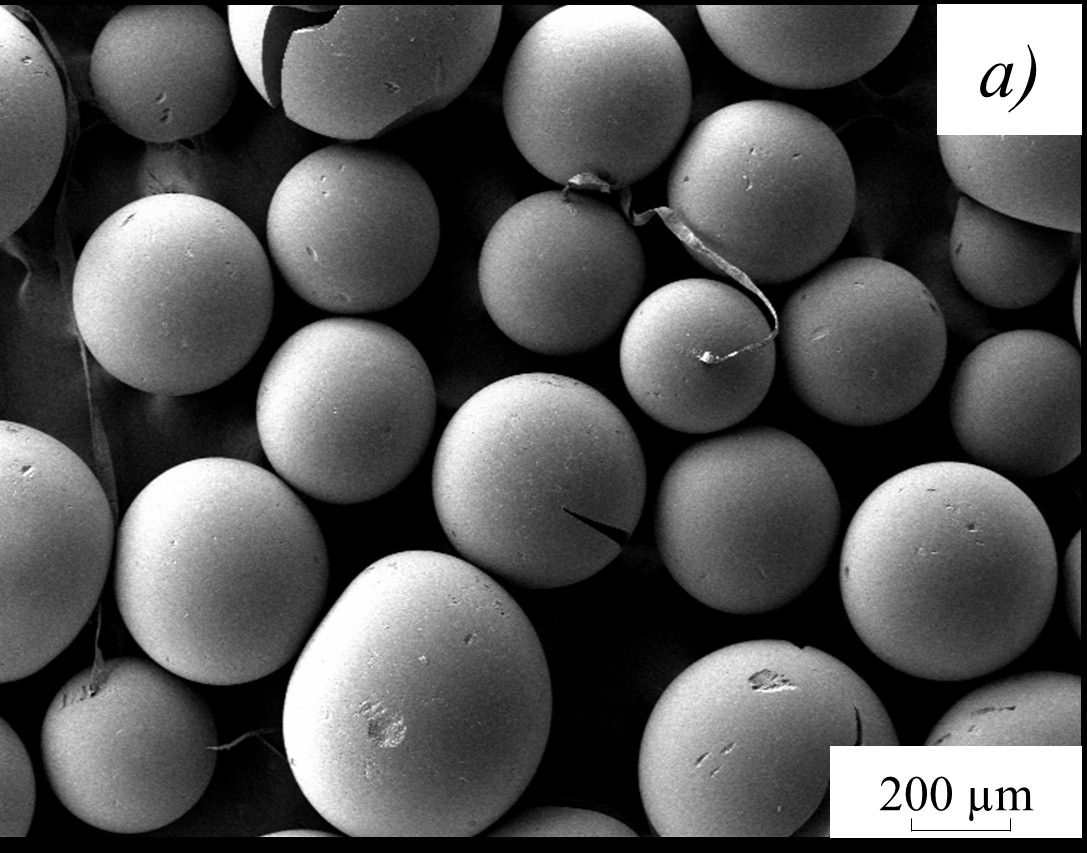
The kinetic studies of AV-17×8 strongly basic anionite’s oxidative destruction using the Fenton reaction have been carried out. The effect of the process’s temperature and the concentration of catalysts of iron(II) sulfate or copper(II) sulfate on the oxidation of anion-exchange resin with hydrogen peroxide is estimated. With an increase in temperature in the range of 323–348 K, a regular increase in the effective rate constant of oxidative anionite destruction is observed when using iron(II) sulfate by 1.5 times, and when using copper(II) sulfate – by 22 times. It was found that the obtained values of the activation energy of the anion exchanger’s oxidation with the addition of copper(II) sulfate are 124.3–115.7 kJ/mol and are characteristic of the process proceeding in the kinetic region. The nature of the change in the surface morphology of the anionite granules in the process of oxidative decomposition has been revealed.
Keywords
Full Text:
PDFReferences
Smolnikov MI, Markov VF, Maskaeva LN, Bobylev AE, Mokrousova OA. Utilization problems of spent ion-exchange resins of nuclear power plants. Butlerov Com-mun. 2017;49(3):119–134. doi:jbc-01/17-49-3-119
Wang J, Wan Z. Treatment and disposal of spent radioactive ion-exchange resins produced in the nuclear industry. Prog Nucl Energy. 2015;78:47–55. doi:10.1016/j.pnucene.2014.08.003
Babuponnusami A., Muthukumar K. A review on Fenton and improvements to the Fenton process for wastewater treatment. J Environ Chem Eng. 2014;2(1):557–572. doi:10.1016/j.jece.2013.10.011
Kuznetsov AE, Knyazev OV, Maraev IY, Manakov MN. Bio-technological destruction of ion exchange resins. Biotech-nol. 2000;16(1):66–77.
Zahorodna M, Bogoczek R, Oliveros E, Braun AM. Applica-tion of the Fenton process to the dissolution and minerali-zation of ion exchange resins. Catal Today. 2007;129(1–2):200–206. doi:10.1016/j.cattod.2007.08.014
Gunale TL, Mahajani VV, Wattal PK, Srinivas C. Liquid phase mineralization of gel-type anion exchange resin by a hybrid process of Fenton dissolution followed by sonication and wet air oxidation. Asia-Pacific J Chem Eng. 2009;4(1):90–98. doi:10.1002/apj.214
Gunale TL, Mahajani VV, Wattal PK, Srinivas C. Studies in liquid phase mineralization of cation exchange resin by a hybrid process of Fenton dissolution followed by wet oxida-tion. Chem Eng J. 2009;148(2–3):371–377. doi:10.1016/j.cej.2008.09.018
Wan Z, Xu L, Wang J. Disintegration and dissolution of spent radioactive cationic exchange resins using Fenton-like oxidation process. Nucl Eng Des. 2015;291:101–108. doi:10.1016/j.nucengdes.2015.05.009
Wan Z, Xu L, Wang J. Treatment of spent radioactive anion-ic exchange resins using Fenton-like oxidation process. Chem Eng J. 2016;284:733–740. doi:10.1016/j.cej.2015.09.004
de Araujo LG, Marumo JT. Reaction of ion exchange resins with fenton’s reagent. Environments – MDPI. 2018;5(11):1–10. doi:10.3390/environments5110123
Xu L, Meng X, Li M, Li W, Sui Z, Wang J, Yang J. Dissolution and degradation of nuclear grade cationic exchange resin by Fenton oxidation combining experimental results and DFT calculations. Chem Eng J. 2019;361:1511–1523. doi:10.1016/j.cej.2018.09.169
Huang CP, Tsai MT, Li YJ, Huang YH, Chung TY. Oxidative dissolution of cation ion exchange resin by the Fenton pro-cess using a fluidized bed reactor. Prog Nucl Energy. 2020;125:1–8. doi:10.1016/j.pnucene.2020.103377
Feng W, Li J, An H, Wang Y. Degradation of spent radioac-tive ion exchange resins and its mechanisms by fenton pro-cess. J Renew Mater. 2020;8(10):1283–1293. doi:10.32604/jrm.2020.011000
Hafeez MA, Jeon J, Hong S, Hyatt N, Heo J, Um W. Fenton-like treatment for reduction of simulated carbon-14 spent resin. J Environ Chem Eng. 2021;9(1):1–9. doi:10.1016/j.jece.2020.104740
Kozlova MM, Markov VF, Maskaeva LN, Smol’nikov MI, Sav-inykh SD. Kinetics of the Oxidative Degradation of KU-2×8 Cation-Exchange Resin Using Hydrogen Peroxide. Russ J Phys Chem A. 2020;94(12):2450–2458. doi:10.1134/S0036024420120146
Pilipenko AT, Pyatnitsky IV. Analiticheskaya khimiya [Ana-lytical chemistry]. Moscow: Chemistry, 1990. 480 p. Rus-sian.
Dyachenko AN, Shagalov VV. Khimicheskaya kinetika get-erogennykh protsessov [Chemical kinetics heterogeneous processes]. Tomsk: Tomsk Poly Technical University, 2014. 102 p. Russian.
DOI: https://doi.org/10.15826/chimtech.2021.8.4.06
Copyright (c) 2021 M. M. Kozlova, V. F. Markov, L. N. Maskaeva

This work is licensed under a Creative Commons Attribution 4.0 International License.
Chimica Techno Acta, 2014–2025
eISSN 2411-1414
Copyright Notice






