Synthesis and analgesic activity evaluation of derivatives of 2-[(1,4-dioxo-1-amino-4-arylbutyl-2-en-2-yl)amino]-4,5,6,7-tetrahydrobenzo[b]thiophene-3-carboxylic acid
Abstract
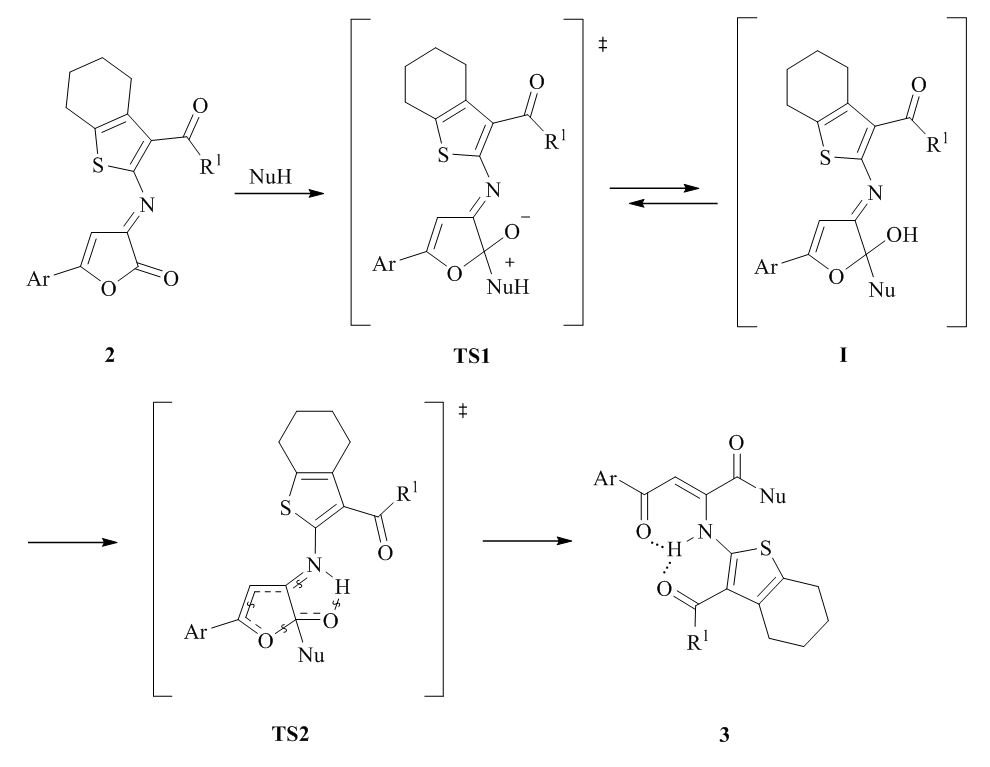
The synthesis of new derivatives of 2-[(1,4-dioxo-1-amino-4-arylbutyl-2-en-2-yl)amino]-4,5,6,7-tetrahydrobenzo[b]thiophene-3-carboxylic acid is described. Starting 2-{[5-aryl-2-oxofuran-3(2H)-ylidene]amino}thiophene-3-carboxylic acids were obtained by intramolecular cyclisation of substituted 4-aryl-4-oxo-2-thienylaminobut-2-enoic acids in acetic anhydride. New derivatives of 2-[(1,4-dioxo-1-amino-4-arylbutyl-2-en-2-yl)amino]-4,5,6,7-tetrahydrobenzo[b]thiophene-3-carboxylic acids were obtained via decyclization reaction of 2-{[5-aryl-2-oxofuran-3(2H)-ylidene]amino}thiophene-3-carboxylic acids. The structure of the compounds obtained was confirmed by the 1H and 13C NMR spectroscopy, IR spectrometry and elemental analysis methods. Analgesic activity of new compounds has been studied by the “hot plate” method on outbred white mice of both sexes with intraperitoneal injection. It was found that derivatives of 2-[(1,4-dioxo-1-amino-4-arylbutyl-2-en-2-yl)amino]-4,5,6,7-tetrahydrobenzo[b]thiophene-3-carboxylic acid possess analgesic effect exceeding the effect of the comparison drug metamizole.
Keywords
Full Text:
PDFReferences
Thomas J, Jecic A,Vanstreels E, van Berckelaer L, Romagnoli R, Dehaen W, Liekens S, Balzarini J. Pronounced anti-proliferative activity and tumor cell selectivity of 5-alkyl-2-amino-3-methylcarboxylate thiophenes. Eur J Med Chem. 2017;132;219–235. doi: 10.1016/j.ejmech.2017.03.044
Regal MKA, Shaban SS, El‐Metwally SA. Facile Synthesis and Antimicrobial Activity of 5‐Amino‐3‐methyl‐1‐phenyl‐1H‐thieno[3,2‐c]pyrazole‐6‐carbonitrile and Their Derivatives. J Heterocyclic Chem. 2018;56(1);226–233. doi: 10.1002/jhet.3399
Vasileva AYu, Vaganov VYu, Shipilovskikh SA, Rubtsov AE. Chemistry of Iminofurans: XV. Decyclization of Ethyl 2-[5-Aryl-2-oxofuran-3(2H)-ylideneamino]-4,5,6,7-tetrahydro-1-benzothiophene-3-carboxylates by the Action of Secondary Amines. Russ J Org Chem. 2018;54(4);582–587. doi: 10.1134/s1070428018040115
Baravkar SB, Sachin B, Wagh MA, Nawale LU, Choudhari AS, Bhansali S, Sarkar D, Sanjayan GJ. Design and Synthesis of 2‐Amino‐thiophene‐proline‐conjugates and Their Anti‐tubercular Activity against Mycobacterium Tuberculosis H37Ra. Chem Select. 2019;4(9);2851–2857. doi: 10.1002/slct.201803370
Puthran D, Poojary B, Purushotham N, Harikrishna N, Nayak SG, Kamat V. Synthesis of novel Schiff bases using 2-Amino-5-(3-fluoro-4-methoxyphenyl)thiophene-3-carbonitrile and 1,3-Disubstituted pyrazole-4-carboxaldehydes derivatives and their antimicrobial activity. Heliyon. 2019;5(8);e02233. doi: 10.1016/j.heliyon.2019.e02233
Rossetti A, Bono N, Candiani G, Meneghetti F, Roda G, Sacchetti A. Synthesis and Antimicrobial Evaluation of Novel Chiral 2-Amino-4,5,6,7-tetrahydrothieno[2,3-c]pyridine Derivatives. Chem Biodivers. 2019;16(6);e1900097. doi: 10.1002/cbdv.201900097
Zhdankin VV, Puterova Z, Krutošíková A, Végh D. Gewald reaction: synthesis, properties and applications of substituted 2-aminothiophenes. Arkivoc. 2010;2010(1);209–246. doi: 10.3998/ark.5550190.0011.105
Javadi F, Tayebee R. Preparation and characterization of ZnO/nanoclinoptilolite as a new nanocomposite and studying its catalytic performance in the synthesis of 2-aminothiophenes via Gewald reaction. Microporous Mesoporous Mats. 2016;231;100–109. doi: 10.1016/j.micromeso.2016.05.025
Akbarzadeh A, Dekamin MG. A facile and environmentally benign polyethylene glycol 600-mediated method for the synthesis of densely functionalized 2-aminothiophene derivatives under ultrasonication. Green Chem Lett Rev. 2017;10(4);315–323. doi: 10.1080/17518253.2017.1380234
Kizimova IA, Igidov NM, Kiselev MA, Dmitriev MV, Chashchina SV, Siutkina AI. Synthesis of new 2-aminopyrrole derivatives by reaction of furan-2,3-diones 3-acylhydrazones with CH-nucleophiles. Russ J Gen Chem. 2020;90(2);182–186. doi:10.1134/S1070363220020036
Kizimova IA, Igidov NM, Kiselev MA, Syutkina AI, Ivanov DV. Reactions of N'-[2-Oxo-5-R-furan-3(2H)-ylidene]acylhydrazides with Primary and Secondary Alcohols. Russ J Gen Chem. 2020;90(5);815–821. doi:10.1134/S1070363220050096
Siutkina AI, Igidov NM, Kizimova IA. Synthesis and Properties of Alkyl 2-[2-(Diarylmethylidene)hydrazinyl]-5,5-dimethyl-4-oxohex-2-enoates. Russ J Org Chem. 2020;56(4);649–653. doi:10.1134/S1070428020040132
Zykova SS, Kizimova IA, Syutkina AI, Toksarova YuS, Igidov NM, Ibishov DF, Boichuk SV, Dunaev PD, Galembikova AR. Synthesis and Cytostatic Activity of (E)-Ethyl-2-Amino-5-(3,3-Dimethyl-4-Oxobutyliden)-4-Oxo-1-(2-Phenylaminobenzamido)-4,5-Dihydro-1H-pyrrol-3-Carboxylate. Pharm Chem J. 2020;53(10);895–898. doi:10.1007/s11094-020-02096-z
Shipilovskikh SA, Rubtsov AE. Recyclization of 3-(Thiophen-2-yl)imino-3H-furan-2-ones under the Action of Cyanoacetic Acid Derivatives. Russ J Gen Chem. 2020;5;809–814. doi:10.1134/S1070363220050084
Shipilovskikh SA, Rubtsov AE. One-Pot Synthesis of Thieno[3,2-e]pyrrolo[1,2-a]pyrimidine Derivative Scaffold: A Valuable Source of PARP-1 Inhibitors. J Org Chem. 2019;84(24);15788–15796. doi: 10.1021/acs.joc.9b00711
Shipilovskikh SA, Rubtsov AE. Decyclization of-2-[5-(4-chlorophenyl)-2-oxofuran-3(2H)-ylideneamino]-4,5,6,7-tetrahydrobenzo[b]thiophene-3-carboxamide upon treatment with aliphatic alcohols. Russ Chem Bull. 2014;63(9);2205–2207. doi:10.1007/s11172-014-0722-4
Shipilovskikh SA, Rubtsov AE. Iminofurans chemistry. Decyclization of ethyl 2-[2-oxo-5-phenylfuran-3(2H)-ylideneamino]-4,5,6,7-tetrahydrobenzo[b]thiophene-3-carboxylate under the action of aliphatic amines. Russ J Org Chem. 2014;50(2);298–300. doi:10.1134/S1070428014020286v
Baughman BM, Jake SP, DuBois RM, Boyd VA,White SW, Webb TR. Identification of influenza endonuclease inhibitors using a novel fluorescence polarization assay. ACS Chem Biol. 2012;7(3);526–534. doi:10.1021/cb200439z
de Melo EB, Ferreira MM. Four-dimensional structure-activity relationship model to predict HIV-1 integrase strand transfer inhibition using LQTA-QSAR methodology. J Chem Inf Model. 2012;52(7);1722–1732. doi: 10.1021/ci300039a
Deore RR, Chen GS, Chen CS, Chang PT, Chuang MH, Chern TR, Wang HC, Chern JW. 2-Hydroxy-1-oxo-1,2-dihydroisoquinoline-3-carboxylic acid with inbuilt beta-N-hydroxy-gamma-keto-acid pharmacophore as HCV NS5B polymerase inhibitors. Curr Med Chem. 2012;19(4);613–624. doi:10.2174/092986712798918833
Kowalinski E, Zubieta C, Wolkerstorfer A, Szolar OH, Ruigrok RW, Cusack S. Structural analysis of specific metal chelating inhibitor binding to the endonuclease domain of influenza pH1N1 (2009) polymerase. PLoS Pathog. 2012;8(8);e1002831. doi:10.1371/journal.ppat.1002831
Sharma H, Sanchez TW, Neamati N, Detorio M, Schinazi RF, Cheng X, Buolamwini JK. Synthesis, docking, and biological studies of phenanthrene beta-diketo acids as novel HIV-1 integrase inhibitors. Bioorg Med Chem Lett. 2013;23(22);6146–6151. doi:10.1016/j.bmcl.2013.09.009
Denisova EI, Shipilovskikh SA, Makhmudov RR, Rubtsov AE. Search of analgesic activity of N-substituted 2-((3-R-4,5,6,7-tetrahydrobenzo[b]thiophen-2-yl)amino)-4-oxo-4-phenylbut-2-enamides. AIP Conf Proc. 2020;2280(1);040013. doi:10.1063/5.0018515
Kizimova IA, Igidov NM, Dmitriev MV, Chashchina SV, Makhmudov RR, Siutkina AI. Synthesis, Structure, and Biological Activity of 4-R-4-Oxo-2-[2-(phenylamino)benzoyl]hydrazinylidene-N-hetarylbutanamides. Russ J Gen Chem. 2019;89(12);2345–2352. doi:10.1134/S107036321912003X
Shipilovskikh SA, Makhmudov RR, Balandina SY, Rubtsov AE. Search of antimicrobial activity in a series of substituted 4-aryl-4-oxo-2-tienilaminobut-2-enoic acids. AIP Conf Proc. 2020;2280(1);030018. doi:10.1063/5.0018494
Siutkina AI, Igidov NM, Dmitriev MV, Makhmudov RR, Novikova VV. Synthesis and Biological Activity of N-Aryl(alkyl)-2-[2-(9H-fluoren-9-ylidene)hydrazinylidene]-5,5-dimethyl-4-oxohexanamides. Russ J Gen Chem. 2019;89(7);1388–1393. doi:10.1134/S1070363219070065
Shipilovskikh SA, Shipilovskikh DA, Rubtsov AE. Chemistry of iminofurans. Recyclization of ethyl 2-[2-oxo-5-phenylfuran-3(2H)-ylideneamino]-4,5,6,7-tetrahydro-1-benzothiophene-3-carboxylate in reaction with amines. Russ J Org Chem. 2017;53(1);137–140. doi:10.1134/S1070428017010274
Shipilovskikh SA, Rubtsov AE, Zalesov VV. Chemistry of iminofurans 3. *Synthesis and intramolecular cyclization of (Z)-4-aryl-2-[3-(ethoxycarbonyl)-4,5,6,7-tetrahydrobenzo[b]thiophen-2-ylamino]-4-oxobuten-2-oic acids. Chem Heterocycl Compd. 2009;45(6);658–661. doi:10.1007/s10593-009-0334-3
Shipilovskikh SA, Makhmudov RR, Lupach DY, Pavlov PT, Babushkina EV, Rubtsov AE. Synthesis and Analgesic Activity of Substituted 4-(Het)aryl-4-oxo-2-thienylaminobut-2-enoic Acids. Pharm Chem J. 2013;47(7);366–370. doi:10.1007/s11094-013-0960-z
Shipilovskikh SA, Rubtsov AE. Synthesis of substituted 2-((2-oxofuran-3(2H)-ylidene)amino)-4,5,6,7-tetrahydrobenzo[b]thiophene-3-carboxamide. AIP Conf Proc. 2020;2280(1);030016. doi:10.1063/5.0018486
Eddy NB, Leimbach DJ. Synthetic analgesics. II. Dithienylbutenyl- and dithienylbutylamines. J Pharmacol Exp Ther. 1953;107(3);385–93.
Mironov AN. Guidelines for conducting preclinical studies of drugs. Part one. M.: Grif and K, 2012. 944 p. Russian.
Belenky ML. Elements of quantitative evaluation of the pharmacological effect. 2nd ed. Leningrad: Medgiz,1963. 146 p. Russian.
Kozlov AP, Sychev DI. Chemistry of oxalyl derivatives of methylketones. 45. The effect of specific solvation on the kinetics of the reaction of 5-aryl-2,3-dihydrofuran-2,3-diones with aromatic amines in dioxane. Russ J Org Chem. 1986;22(8);1756–1762. Russian.
Kozlov AP, Sychev DI, Andreychikov YS. Chemistry of oxalyl derivatives of methylketones. 42. Disclosure of the cycle of 5-aryl-2,3-dihydrofuran-2,3-diones under the action of aromatic amines in toluene. The effect of substituents in nucleophilic reagents and substrate on the rate of non-catalytic reaction. Russ J Org Chem. 1985;21(10);2147–2154. Russian.
Kozlov AP, Sazhnev SS, Kozlova GA, Andreichikov YS. Effect of the solvent and substituent in the nucleophile on the kinetics of noncatalytic and acetic or diphenylphosphinic acid-catalyzed reaction of 4-methyl-5-phenyl-2,3-dihydrofuran-2,3-dione with aromatic amines. Russ J Org Chem. 2000;36(3);417–421.
Shurov SN, Porvintsev IB, Kosvintseva LS, Andreichikov YS. Five-membered 2,3-Dioxoheterocycles. XLIV. Synthesis and Nucleophilic Reactions of 5-(β-Styryl)-2,3-dihydro-2.3-furandione. Russ J Org Chem.1997;33(8);1116–1124.
DOI: https://doi.org/10.15826/chimtech.2021.8.4.04
Copyright (c) 2021 Alena I. Siutkina, Ramiz R. Makhmudov, Daria A. Shipilovskikh

This work is licensed under a Creative Commons Attribution 4.0 International License.
Chimica Techno Acta, 2014–2025
eISSN 2411-1414
Copyright Notice







