Deoxydichlorination of aldehydes catalyzed by Diphenyl sulfoxide
Abstract
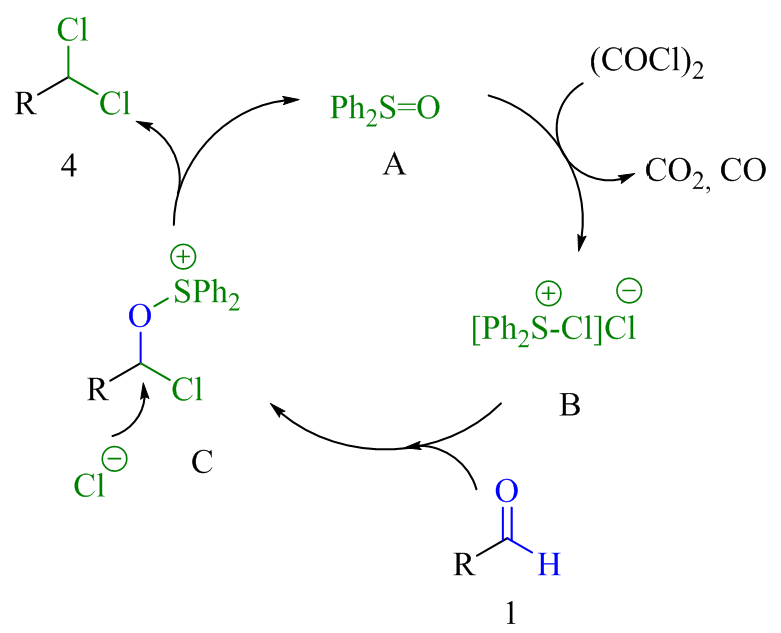
The diphenyl sulfoxide-catalyzed conversion of aldehydes to 1,1-dichlorides is reported. The reaction proceeds via a sulfurous (IV)-catalysis manifold in which diphenyl sulfoxide turnover is achieved using oxalyl chloride as a consumable reagent.
Keywords
Full Text:
PDFReferences
Huy PH. Lewis base catalysis promoted nucleophilic substitutions – recent advances and future directions. Eur J Org Chem. 2020;(1):10–27. doi:10.1002/ejoc.201901495
Beddoe RH, Sneddon HF, Denton RM. The catalytic Mitsunobu reaction: a critical analysis of the current state-of-the-art. Org Biomol Chem. 2018;16(42):7774–7781. doi:10.1039/C8OB01929K
Shipilovskikh SA, Rubtsov AE. Dehydration of oxime to nitriles. AIP Conf Proc. 2019;2063:030019. doi:10.1063/1.5087327
Huy PH, Hauch T, Filbrich I. Lewis base catalyzed nucleophilic substitutions of alcohols. Synlett. 2016;27(19):2631–2636. doi:10.1055/s-0036-1588633
Kohlmeyer C, Schäfer A, Huy PH, Hilt G. Formamide-catalyzed nucleophilic substitutions: mechanistic insight and rationalization of catalytic. ACS Catal. 2020;10(19):11567–11577. doi:10.1021/acscatal.0c03348
Shipilovskikh SA, Vaganov VY, Denisova EI, Rubtsov AE, Malkov AV. Dehydration of amides to nitriles under conditions of a catalytic appel reaction. Org Lett. 2018;20(3):728–731. doi:10.1021/acs.orglett.7b03862.
Huy PH, Mbouhom C. Formamide catalyzed activation of carboxylic acids – versatile and cost-efficient amidation and esterification. Chem Sci. 2019;10:7399–7406. doi:10.1039/C9SC02126D
Motsch S, Schütz C, Huy PH. Systematic evaluation of sulfoxides as catalysts in nucleophilic substitutions of alcohols. Eur J Org Chem. 2018:4541–4547. doi:10.1002/ejoc.201800907
Huy PH, Filbrich I. A general catalytic method for highly cost- and atom-efficient nucleophilic substitutions. Chem Eur J. 2018;24:7410. doi:10.1002/chem.201800588
Fukazawa Y, Vaganov VY, Shipilovskikh SA, Rubtsov AE, Malkov AV. Stereoselective synthesis of atropisomeric bipyridine N,N′-dioxides by oxidative coupling. Org Lett. 2019;21(12):4798–4802. doi:10.1021/acs.orglett.9b01687
Takeda T, Endo Y, Reddy ACS, Sasaki R, Fujiwara T. Transformation of ketones into 1-chloro and 1,1-dichloro-1-alkenes by means of a polychloromethane-titanocene(II) system. Tetrahedron. 1999;55:2475. doi:10.1016/S0040-4020(99)00021-6
Takeda T, Sasaki R, Fujiwara T. Carbonyl Olefination by Means of a gem-Dichloride−Cp2Ti[P(OEt)3]2 System. J Org Chem. 1998;63(21):7286–7288. doi:10.1021/jo980724h
Huy PH. Formamide catalysis facilitates the transformation of aldehydes into geminal dichlorides. Synthesis. 2019;51(12):2474–2483. doi:10.1055/s-0037-1611798
An J, Tang X, Moore J, Lewis W, Denton RM. Phosphorus(V)-catalyzed dichlorination reactions of aldehydes. Tetrahedron. 2013;69:8769–8776. doi:10.1016/j.tet.2013.07.100
Concellón JM, Rodríguez-Solla H, Díaz P, Llavona R. The first sequential reaction promoted by manganese: complete stereoselective synthesis of (E)-α,β-unsaturated esters from 2,2-dichloroesters and aldehydes. J Org Chem. 2007;72:4396. doi:10.1021/jo070209w
Concellón JM, Rodríguez-Solla H, de Amo V, Díaz P. Stereoselective olefination reactions promoted by rieke manganese. Synthesis. 2009;15:2634–2645. doi:10.1055/s-0029-1216880
Oudeyer S, Leonel E, Paugam JP, Nédélec JY. Formation of epoxides and N-arylaziridines via a simple Mg-Barbier reaction in DMF. Tetrahedron. 2014;70:919–923. doi:10.1016/j.tet.2013.12.016
Zhou YY, Uyeda C. Reductive cyclopropanations catalyzed by dinuclear nickel complexes. Angew Chem Int Ed. 2016;55:3171–3175. doi:10.1002/anie.201511271
Durán-Peña MJ, Flores-Giubi ME, Botubol-Ares JM, Harwood LM, Collado IG, MacÍas-Sánchez AJ, Hernández-Galán R. Chemoselective and stereoselective lithium carbenoid mediated cyclopropanation of acyclic allylic alcohols. Org Biomol Chem. 2016;14(9):2731–2741. doi:10.1039/c5ob02617b
Barrero AF, Herrador MM, Del Moral JFQ, Arteaga P, Akssira M, El Hanbali F, Arteaga JF, Diéguez HR, Sánchez EM. Couplings of benzylic halides mediated by titanocene chloride: Synthesis of bibenzyl derivatives. J Org Chem. 2007;72(6):2251–2254. doi:10.1021/jo062492p
Eisch JJ, Qian Y, Rheingold AL. Nickel(II)-carbene intermediates in reactions of geminal dihaloalkanes with nickel(0) reagents and the corresponding carbene capture as the phosphonium ylide. Eur J Inorg Chem. 2007;(11):1576–1584. doi:10.1002/ejic.200601106
Giannerini M, Fañanas-Mastral M, Feringa BL. Z-Selective Copper-Catalyzed Asymmetric Allylic Alkylation with Grignard Reagents. J Am Chem Soc. 2012;134(9):4108–4111. doi:10.1021/ja300743t
Li H, Müller D, Guénée L, Alexakis A. Copper-catalyzed enantioselective synthesis of axially chiral allenes. Org Lett 2012;14(23):5880–5883. doi:10.1021/ol302790e
Li H, Grassi D, Guénée L, Bürgi T, Alexakis A. Copper-catalyzed propargylic substitution of dichloro substrates: Enantioselective synthesis of trisubstituted allenes and formation of propargylic quaternary stereogenic centers. Chem Eur J. 2014;20(50):16694–16706. doi:10.1002/chem.201404668
Brześkiewicz J, Loska R, Makosza M. α-Chlorobenzylation of nitroarenes via vicarious nucleophilic substitution with benzylidene dichloride: Umpolung of the friedel-crafts reaction. J Org Chem. 2018;83(15):8499–8508. doi:10.1021/acs.joc.8b01091
Nilewski C, Carreira EM. Recent advances in the total synthesis of chlorosulfolipids. Eur J Org Chem. 2012;(9):1685–1698. doi: 10.1002/ejoc.201101525
Chung WJ, Vanderwal CD. Stereoselective halogenation in natural product synthesis. Angew Chem Int Ed. 2016;55:4396–4434.
DOI: https://doi.org/10.15826/chimtech.2021.8.4.08
Copyright (c) 2021 I.A. Gorbunova, D.A. Shipilovskikh, S.A. Shipilovskikh

This work is licensed under a Creative Commons Attribution 4.0 International License.
Chimica Techno Acta, 2014–2025
eISSN 2411-1414
Copyright Notice







