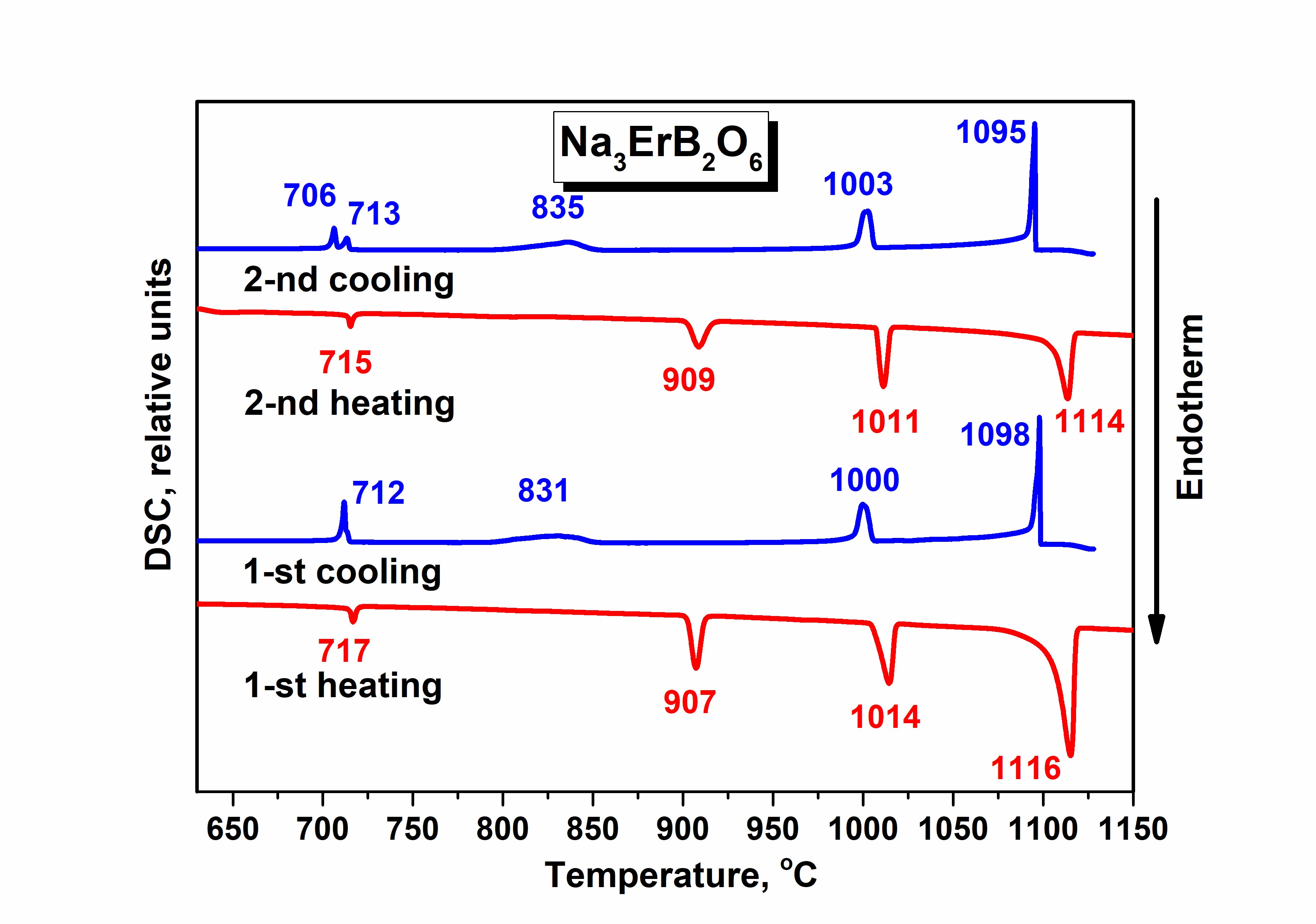
Synthesis, crystal structure, and thermal stability of double borate Na3ErB2O6
Abstract
Keywords
Full Text:
PDFReferences
Mutailipu M, Poeppelmeier KR, Pan S. Borates: A Rich Source for Optical Materials. Chem Rev. 2021;121:1130–1202. doi:10.1021/acs.chemrev.0c00796
Yang SH, Xue H, Guo SP. Borates as promising electrode materials for rechargeable batteries. Coord Chem Rev. 2021;427. doi:10.1016/j.ccr.2020.213551
Leonyuk NI, Maltsev VV, Volkova EA. Crystal chemistry of high-temperature borates. Molecules. 2020;25. doi:10.3390/MOLECULES25102450
Topnikova AP, Belokoneva EL. The structure and classifica-tion of complex borates. Russ Chem Rev. 2019;88:204–228. doi:10.1070/rcr4835
Mutailipu M, Zhang M, Yang Z, Pan S. Targeting the Next Generation of Deep-Ultraviolet Nonlinear Optical Materi-als: Expanding from Borates to Borate Fluorides to Fluo-rooxoborates. Acc Chem Res. 2019;52:791–801. doi:10.1021/acs.accounts.8b00649
Becker P. Borate materials in nonlinear optics. Adv Mater. 1998;10:979–992. doi:10.1002/(SICI)1521-4095(199809)10:13<979::AID-ADMA979>3.0.CO;2-N
Chen C, Wu Y, Li R. The development of new NLO crystals in the borate series. J Cryst Growth. 1990;99:790–798. doi:10.1016/S0022-0248(08)80028-0
Kovtunets EV, Subanakov AK, Bazarov BG. Synthesis, struc-ture and luminescent properties of the new double borate K3Eu3B4O12. Kondens Sredy Mezhfaznye Granitsy. 2020;22:219–224. doi:10.17308/kcmf.2020.22/2823
Subanakov AK, Kovtunets EV, Bazarov BG, Dorzhieva SG, Bazarova JG. New double holmium borates: Rb3HoB6O12 and Rb3Ho2B3O9. Solid State Sci. 2020;105. doi:10.1016/j.solidstatesciences.2020.106231
Subanakov AK, Kovtunets EV, Bazarov BG, Pugachev AM, Sofich DO, Bazarova JG. Exploration of structural, thermal and vibrational properties of new noncentrosymmetric dou-ble borate Rb3Tm2B3O9. Solid State Sci. 2021;120. doi:10.1016/j.solidstatesciences.2021.106719
Xia M, Shen S, Lu J, Sun Y, Li R. K3Li3Gd7(BO3)9: A New Gadolinium-Rich Orthoborate for Cryogenic Magnetic Cool-ing. Chem – A Eur J. 2018;24:3147–3150. doi:10.1002/chem.201705669
Xia M, Zhai K, Lu J, Sun Y, Li RK. Orthoborates LiCdRE5(BO3)6 (RE = Sm–Lu and Y) with Rare-Earth Ions on a Triangular Lattice: Synthesis, Crystal Structure, and Opti-cal and Magnetic Properties. Inorg Chem. 2017;56:8100–8105. doi:10.1021/acs.inorgchem.7b00756
Xia M-J, Li RK. A new quaternary rare earth borate, CsLi2Gd4(BO3)5. Acta Crystallogr Sect E Struct Reports Online. 2007;63. doi:10.1107/S1600536807036586
Chen C, Wu Y, Li R. The development of new NLO crystals in the borate series. J Cryst Growth. 1990;99:790–798. doi:10.1016/S0022-0248(08)80028-0
Chen C, Li R. The anionic group theory of the non-linear optical effect and its applications in the development of new high-quality NLO crystals in the borate series. Int Rev Phys Chem. 1988;8:65–91. doi:10.1080/01442358909353223
Qin F, Li RK. Predicting refractive indices of the borate opti-cal crystals. J Cryst Growth. 2011;318:642–644. doi:10.1016/J.JCRYSGRO.2010.08.037
Yu H, Pan Z, Zhang H, Wang J. Recent advances in self-frequency-doubling crystals. J Mater. 2016;2:55–65. doi:10.1016/j.jmat.2015.12.001
Jia Z, Xia M. Congruent melt terbium-rich borate Na2Tb2B2O7: Synthesis, crystal structure, optical and mag-netic properties.J Alloys Compd. 2018;743:537–542. doi:10.1016/j.jallcom.2018.02.031
Shan F, Zhang G, Yao J, Xu T, Zhang X, Fu Y, et al. Growth, structure, and optical properties of a self-activated crystal: Na2Nd2O(BO3)2. Opt Mater (Amst). 2015;46:461–466. doi:10.1016/j.optmat.2015.05.004
Nagpure PA, Omanwar SK. Synthesis and photolumines-cence study of rare earth activated phosphor Na2La2B2O7. J Lumin. 2012;132:2088–2091. doi:10.1016/j.jlumin.2012.03.068
Mascetti J, Fouassier C, Hagenmuller P. Concentration quenching of the Nd3+ emission in alkali rare earth borates. J Solid State Chem. 1983;50:204–212. doi:10.1016/0022-4596(83)90189-5
Wang Z, Li H, Cai G, Jin Z. Synthesis, crystal structure, and thermal stability of new borates Na3REB2O6 (RE = Pr, Sm, Eu). Powder Diffr. 2016;31:110–117. doi:10.1017/S0885715616000051
Zhang Y, Chen XL, Liang JK, Xu T. Synthesis and structural study of new rare earth sodium borates Na3Ln(BO3)2 (Ln=Y, Gd). J Alloys Compd. 2002;333:72–75. doi:10.1016/S0925-8388(01)01689-9
Zhang G, Wu Y, Fu P, Wang G, Liu H, Fan G, et al. A new sodium samarium borate Na3Sm2(BO3)3. J Phys Chem Solids. 2001;63:145–9. doi:10.1016/S0022-3697(01)00090-7
Yang Z, Ning Y, Keszler DA. Na3Sc2(BO3)3. Acta Crystallogr Sect E Struct Reports Online. 2006;62:266–268. doi:10.1107/S1600536806036737
Zhou WW, Zhuang RZ, Zhao W, Wang GF, Zhang LZ, Ma JG, et al. Second harmonic generation in Na3Gd2(BO3)3 crystals. Cryst Res Technol. 2011;46:926–930. doi:10.1002/crat.201100077
Shan F, Kang L, Zhang G, Yao J, Lin Z, Xia M, et al. Na3Y3(BO3)4: A new noncentrosymmetric borate with an open-framework structure. Dalt Trans. 2016;45:7205–7208. doi:10.1039/c6dt00950f
Gravereau P, Chaminade JP, Pechev S, Nikolov V, Ivanova D, Peshev P. Na3La9O3(BO3)8, a new oxyborate in the ternary system Na2O–La2O3–B2O3: Preparation and crystal structure. Solid State Sci. 2002. doi:10.1016/S1293-2558(02)01344-4
Zhang J, Zhang G, Li Y, Wu Y, Fu P, Wu Y. Thermophysical properties of a new nonlinear optical Na3La9O3(BO3)8 crys-tal. Cryst Growth Des. 2010;10:4965–4967. doi:10.1021/cg1010743
Coelho AA. Topas: General Profile and Structure Analysis Software for Powder Diffraction Data. Bruker AXS, 2005.
DOI: https://doi.org/10.15826/chimtech.2021.8.4.02
Copyright (c) 2021 Alexey K. Subanakov, Evgeniy V. Kovtunets, Bair G. Bazarov, Jibzema G. Bazarova

This work is licensed under a Creative Commons Attribution 4.0 International License.
Chimica Techno Acta, 2014–2025
eISSN 2411-1414
Copyright Notice







