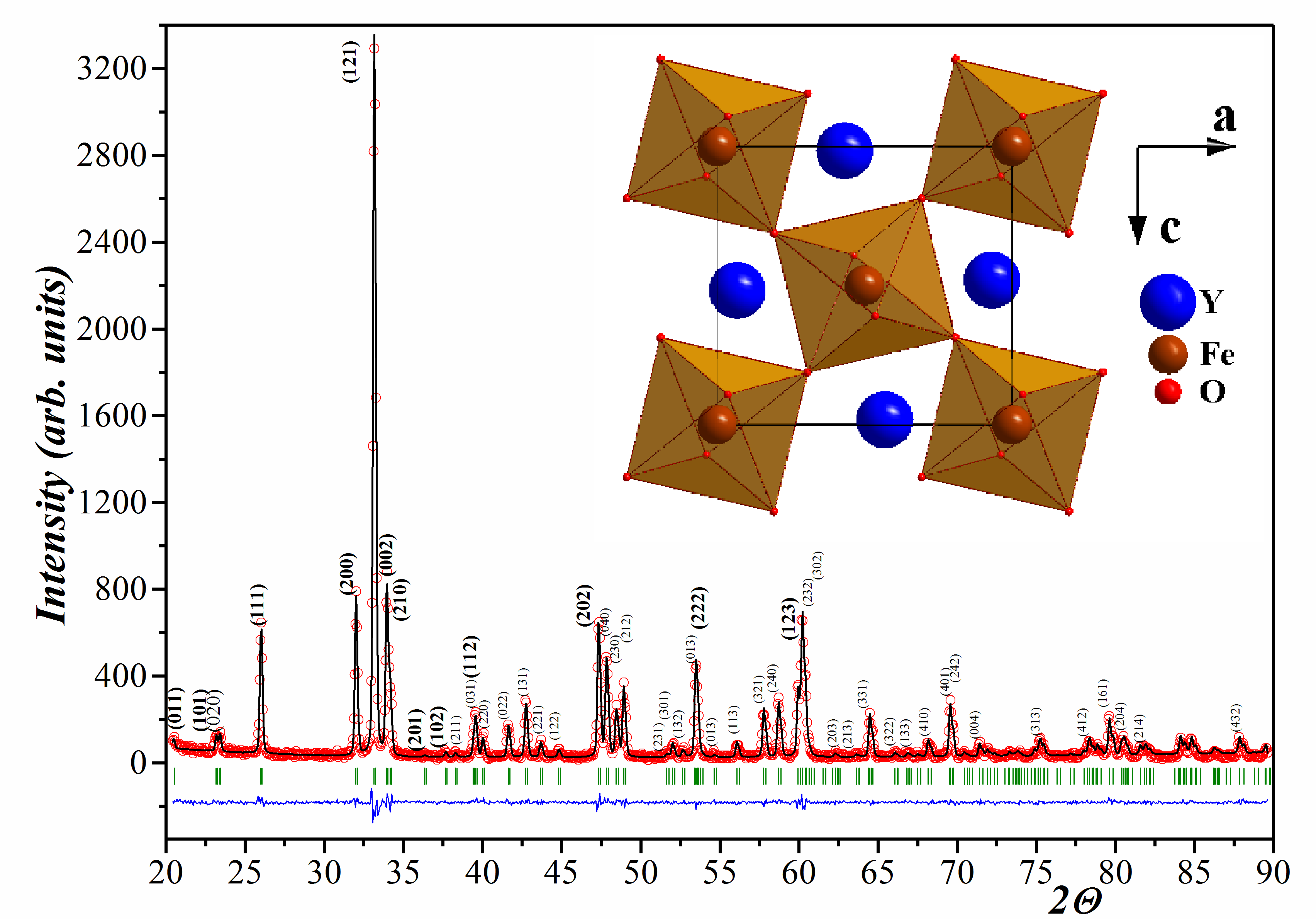
Phase equilibria in the YFeO3 – YСoO3 system in air
Abstract
Keywords
Full Text:
PDFReferences
Rosales-González O, Sánchez-De Jesús F, Cortés-Escobedo CA, Bolarín-Miró AM. Crystal structure and multiferroic behavior of perovskite YFeO3. Ceram Int. 2018; 4:15298-303. doi:10.1016/j.ceramint.2018.05.175
Cheng ZX, Shen H, Xu JY, Liu P, Zhang SJ. Magnetocapacitance effect in nonmultiferroic YFeO3 single crystal. J Appl Phys. 2012;111:034103-1–5. doi:10.1063/1.3681294
Zhang Y, Yang J, Xu J, Gao Q, Hong Z. Controllable synthesis of hexagonal and orthorhombic YFeO3 and their visible-light photocatalytic activity. Mater Lett. 2012;81:1–4. doi:10.1016/j.matlet.2012.04.080
Maiti R, Basu S, Chakravorty D. Synthesis of nanocrystalline YFeO3 and its magnetic properties. J Magn Magn Mater. 2009;321:3274–7. doi:10.1016/j.jmmm.2009.05.061
Lin X, Jiang J, Jin Z, Wang D, Tian Z, Han J, Cheng Z, Ma G. Terahertz probes of magnetic field induced spin reorientation in YFeO3 single crystal. Appl Phys Lett. 2015;106:092403-1–4. doi:10.1063/1.4913998
Addabbo T, Bertocci F, Fort A, Mugnaini M, Shahin L, Vignoli V, Spinicci R, Rocchi S, Gregorkiewitz M. An artificial olfactory system (AOS) for detection of highly toxic gases in air based on YCoO3. Procedia Eng. 2014;87:1095–8. doi:10.1016/j.proeng.2014.11.355
Knížek K, Jirák Z, Hejtmánek J, Veverka M, Maryško M, Maris G, Palstra TTM. Structural anomalies associated with the electronic and spin transitions in LnCoO3. Eur Phys J B. 2005:47:213–20. doi:10.1140/epjb/e2005-00320-3
Zhu Z, Guo J, Jia Y, Hu X. Electronic structure and evolution of spin state in YCoO3. Phys B. 2010;405:359–62. doi:10.1016/j.physb.2009.08.097
Knizek K, Jirak Z, Hejtmanek J, Veverka M, Marysko M, Hauback BC, Fjellvag H. Structure and physical properties of YCoO3–δ at temperatures up to 1000 K. Phys Rev B: Condens Matter Mater Phys. 2006;73:214443. doi:10.1103/PhysRevB.73.214443
Krén E, Pardavi M, Pokó Z, Sváb E, Zsoldos É. Study of the Spin Reorientation in Co- and Cr-Substituted YFeO3. AIP Conf Proc 10. 1973;10:1603–6. doi:10.1063/1.2946858
Pomiro F, Gil DM, Nassif V, Paesano AJr, Gómez MI, Guimpel J, Sánchez RD, Carbonio RE. Weak ferromagnetism and superparamagnetic clusters coexistence in YFe1-xCoxO3 (0
Wei Y, Gui H, Zhao Z, Li J, Liu Y, Xin S, Li X, Xie W. Structure and magnetic properties of the perovskite YCo0.5Fe0.5O3. AIP Adv. 2014;4:127134. doi:10.1063/1.4904811
Imitrovska-Lazova S, Aleksovska S, Tzvetkov P. Synthesis and crystal structure determination of YCo1−xFexO3 (x = 0, 0.33, 0.5, 0.67 and 1) perovskites. J Chem Sci. 2015;127(7):1173–81. doi:10.1007/s12039-015-0878-y
Geller S, Wood EA. Crystallographic studies of perovskite-like compounds. I. Rare earth orthoferrites and YFeO3, YCrO3, YAlO3. Acta Cryst. 1956;9:563-8. doi:10.1107/S0365110X56001571
Buassi-Monroy OS, Luhrs CC, Chávez-Chávez A, Michel CR. Synthesis of crystalline YCoO3 perovskite via sol–gel method. Mater Lett. 2004;58:716–8. doi:10.1016/j.matlet.2003.07.001
Feng G, Xue Y, Shen H, Feng S, Li L, Zhou J, Yang H, Xu D. Sol–gel synthesis, solid sintering, and thermal stability of single-phase YCoO3. Phys Status Solidi A. 2012;209(7):1219–24. doi:10.1002/pssa.201127710
Kimizuka N, Katsura T, Standard free energy of formation of YFeO, Y3Fe5O12, and a new compound YFeO in the Fe-FeO-Y2O3 system at 1200°C. J Solid State Chem. 1975;13:176-81. doi:10.1016/0022-4596(75)90116-4
Kitayama K, Sakaguchi M, Takahara Y, Endo H, Ueki H. Phase equilibrium in the system Y–Fe–O at 1100°C. J Solid State Chem. 2004;177:1933–8. doi:10.1016/j.jssc.2003.12.040
Piekarczyk W, Weppner W, Rabenau A. Dissociation pressure and Gibbs energy of formation of Y3Fe5O12 and YFeO3. Mater Res Bull. 1978;13:1077-83. doi:10.1016/0025-5408(78)90174-5
Tretyakov YuD, Kaul AR, Portnoy VK. Formation of rare earth and yttrium orthferrites: a thermodynamic study. High Temp Sci. 1977;9:61-70.
Jacob KT, Rajitha G. Nonstoichiometry, defects and thermodynamic properties of YFeO3, YFe2O4 and Y3Fe5O12. Solid State Ionics. 2012;224:32-40. doi:10.1016/j.ssi.2012.07.003
Masse DP, MUAN A. Phase Equilibria at Liquidus Temperatures in the System Cobalt Oxide-Iron Oxide-Silica in Air. J Am Ceram Soc. 1965;48:466-9. doi:10.1111/j.1151-2916.1965.tb14800.x
Zhang WW, Chen M. Thermodynamic modeling of the Co–Fe–O system. CALPHAD. 2013;41:76-88. doi:10.1016/j.calphad.2013.02.002
Jung I-H, Decterov SA, Pelton AD, Kim H-M, Kang Y-B. Thermodynamic evaluation and modeling of the Fe–Co–O system. Acta Mater. 2004;52:507–519. doi:10.1016/j.actamat.2003.09.034
Jadhao VG, Singru RM, Rama Rao G, Bahadur D, Rao CNR. Effect of the Rare Earth Ion on the Spin State Equilibria in Perovskite Rare Earth Metal Cobaltates. Yttrium trioxocobaltate(III) and erbium trioxocobaltate(III). J Chem Soc, Faraday Trans 2. 1975;71:1885–93. doi:10.1039/F29757101885
Ahmad I, Akhtar MJ, Siddique M, Iqbal M, Hasan MM. Origin of anomalous octahedral distortions and collapse of magnetic ordering in Nd1-xSrxFeO3 (0
Dasgupta N, Krishnamoorthy R, Jacob KT. Crystal structure and thermal and electrical properties of the perovskite solid solution Nd1-xSrxFeO3-d (0
Raccach PM, Goodenough JB. A localized-electron collective-electron transition in the system (La, Sr)CoO3. J Appl Phys. 1968;39(2):1209-10. doi:10.1063/1.1656227
Yan J-Q, Zhou J-S, Goodenough JB. Bond-length fluctuations and the spin-state transition in LCoO3 (L=La, Pr, and Nd). Phys Rev B. 2004;69:134409-1–6. doi:10.1103/PhysRevB.69.134409
Zhou J-S, Yan J-Q, Goodenough JB. Bulk modulus anomaly in RCoO3 (R=La, Pr, and Nd). Phys. Rev. B. 2005;71:220103-1–4. doi:10.1103/PhysRevB.71.220103
Cavalcante FHM, Carbonari AW, Malavasi RFL, Cabrera-Pasca GA, Saxena RN, Mestnik-Filho J. Investigation of spin transition in GdCoO3 by measuring the electric field gradient at Co sites. J Magn Magn Mater. 2008;320:e32–5. doi:10.1016/j.jmmm.2008.02.033
Shannon RD. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr, Sect A. 1976; 32(5):751–67. doi:10.1107/s0567739476001551
DOI: https://doi.org/10.15826/chimtech.2021.8.1.08
Copyright (c) 2021 A.V. Bryuzgina, A.S. Urusova, I.L. Ivanov, V.A. Cherepanov

This work is licensed under a Creative Commons Attribution 4.0 International License.
Chimica Techno Acta, 2014–2025
eISSN 2411-1414
Copyright Notice






