In situ synthesis, structural chemistry and vibrational spectroscopy of Zn-doped Ca5Mg4(VO4)6
Abstract
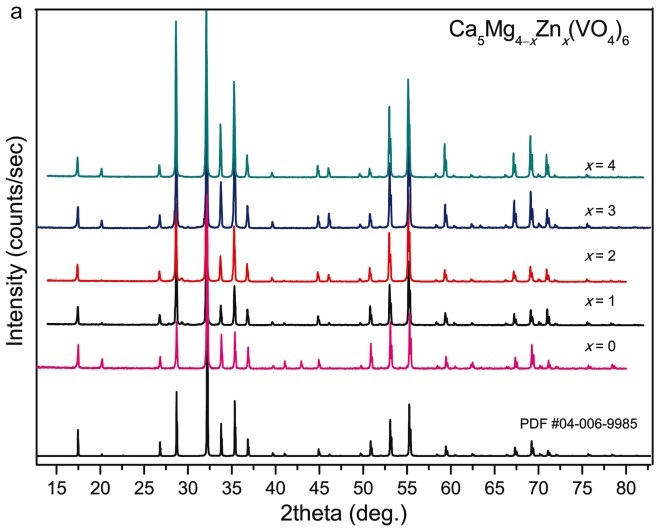
The phase formation of the solid solution Ca5Mg4–xZnx(VO4)6 (0≤x≤4) was studied in situ using differential scanning calorimetry and high-temperature X-Ray powder diffraction (XRPD). XRPD analysis shows the appearance of unavoidable secondary pyrovanadate phases using conventional synthesis methods. The local structure of the solid solution was verified by vibrational spectroscopy. The analysis of the infrared and Raman spectroscopy data allows establishing the main features between vanadate garnets and their isostructural analogs among natural silicates.
Keywords
Full Text:
PDFReferences
Mill BV, Ronniger G, Kabalov YuK. New garnet compounds A32+B22+C4+V25+O12 (A = Ca, Cd; B = Mg, Zn, Co, Ni, Cu, Mn, Cd; C = Ge, Si). Russ J Inorg Chem. 2014;59:1208–1213. doi:10.1134/S0036023614110151
Ishi K, Ikuta Y. Isomorphous substitutions in vanadate garnets. N Jb Miner Abh. 2006;182:157–163. doi:10.1127/0077-7757/2006/0038
Yao GG, Liu P, Zhang HW. Novel series of low-firing microwave dielectric ceramics: Ca5A4(VO4)6 (A2+ = Mg, Zn). J Am Ceram Soc. 2013;96:1691–1693. doi:10.1111/jace.12359
Müller-Buschbaum Hk, von Postel M. Eine weitere Oxovanadat-Phase mit Granatstruktur: Ca5Mg3ZnV6O24. Z Anorg Allg Chem. 1992;615:101–103. doi:10.1002/zaac.19926150920
Krasnenko TI, Zolotukhina LV, Zabolotskaya EV, Svetlakov SV, Dobosh VG. Defect structure and electrical and magnetic properties of calcium manganese vanadium garnets. Inorg Mater. 1999;35:1179–1182.
Ronniger G, Mill BV. Vanadates with a defect garnet structure. Sov Phys Crystallogr. 1973;18:303–307.
Surat LL, Fotiev AA, Dobosh VG. V2O5–MgO–CaO–MnO(Mn2O3) system. Zh Neorg Khim. 1989;34:2924–2928.
Slobodin BV, Fotiev AA, Sharova NG. Phase composition of the CaO–MgO–V2O5 system. Zh Neorg Khim. 1977;23:184–187.
Li B, Zheng JG, Li W. Influence of cobalt ions non-stoichiometry on the microstructure and microwave properties of Ca5Co4(VO4)6 ceramics. Ceram Int. 2017;43:13956–13962. doi:10.1016/j.ceramint.2017.07.127
Li B, Tian J, Qiu L. Crystal structures and microwave dielectric properties of low-firing Ca5Zn4−xMgxV6O24 ceramics. Ceram Int. 2018;44:18250–18255. doi:10.1016/j.ceramint.2018.07.035
Wang D, Xiang HC, Tang Y. Fang L, Khaliq J, Li CC. A low-firing Ca5Ni4(VO4)6 ceramic with tunable microwave dielectric properties and chemical compatibility with Ag. Ceram Int. 2016;42:15094–15098. doi:10.1016/j.ceramint.2016.06.085
Fang L, Xiang F, Su CX, Zhang H. A novel low firing micro-wave dielectric ceramic NaCa2Mg2V3O12. Ceram Int. 2013;39:9779–9783. doi:10.1016/j.ceramint.2013.05.041
Xiang HC, Fang L, Jiang XW, Li CC. Low-firing and micro-wave dielectric properties of Na2YMg2V3O12 ceramic. Ceram Int. 2016;42:3701–3705. doi:10.1016/j.ceramint.2015.10.163
Yao GG, Liu P, Zhao XG, Zhou JP, Zhang HW. Low-temperature sintering and microwave dielectric properties of Ca5Co4(VO4)6ceramics. J Eur Ceram Soc. 2014;34:2983–2987. doi:10.1016/j.jeurceramsoc.2014.03.026
Pavitra E, Raju GSR, Park JY, Wang L, Moon BK, Yu JS. Novel rare-earth-free yellow Ca5Zn3.92In0.08(V0.99Ta0.01O4)6 phosphors for dazzling white light-emitting diodes. Sci Rep. 2015;5:10296. doi:10.1038/srep10296
Li JF, Qiu KH, Li JF, Li W, Yang Q, Li JH. A novel broadband emission phosphor Ca2KMg2V3O12 for white light emitting diodes. Mater Res Bull. 2010;45:598–602. doi:10.1016/j.materresbull.2010.01.014
Song D, Guo CF, Zhao J, Suo H, Zhao XQ, Zhou XJ, Liu GZ. Host sensitized near-infrared emission in Nd3+-Yb3+ Co-doped Na2GdMg2V3O12 phosphor. Ceram Int. 2016;42:12988–12994. doi:10.1016/j.ceramint.2016.05.072
Dhobale AR, Mohapatra M, Natarajan V, Godbole SV. Synthesis and photoluminescence investigations of the white light emitting phosphor, vanadate garnet, Ca2NaMg2V3O12 co-doped with Dy and Sm. J Lumin. 2012;132:293–298. doi:10.1016/j.jlumin.2011.09.004
Leonidova ON, Patrakeev MV, Leonidov IA. Ionic and electronic transport in the garnet-type vanadate Ca2.5Mg2V3O12. J Solid State Electrochem. 2019;23:1083–1088. doi:10.1007/s10008-019-04202-y
Tolkacheva AS, Shkerin SN, Nikonov AV, Pershina SV, Khavlyuk PD, Leonidov II. Electrical and thermal properties of Ca5Mg4−xCox(VO4)6 (0≤x≤4), a promising electrode mate-rial. Mater. Lett. 2021;305:30811. doi:10.1016/j.matlet.2021.130811
Blanzat B, Loriers J. Synthese et etude des proprietes spectrales de monocristaux de vanadate a structure grenat Ca2NaMg2V3O12 active par l'europium trivalent. Mater Res Bull. 1974;9:1647–1654. doi:10.1016/0025-5408(74)90156-1
Dhanaraj G, Byrappa K, Prasad V, Dudley M. (Eds.). Springer Handbook of Crystal Growth. Heidelberg, Germany: Springer-Verlag GmbH; 2010. 1736 p.
Yu YM, Chani VI, Shimamura K, Inaba K, Fukuda T. Growth of vanadium garnet fiber crystals and variations of lattice parameter. J Cryst Growth 1997;177:74–78. doi:10.1016/S0022-0248(97)01070-1
Levina AA, Tadevosyan NO, Petrova SA, Buyanova ES, Morozova MV. Phase formation processes and synthesis of solid solutions in Ca–R–Nb–M–O systems. Chim Techno Acta. 2020;7:17–25. doi:10.15826/chimtech.2020.7.1.03
Klyndyuk AI, Chizhova EA, Shevchenko SV. Spin-state transition in the layered barium cobaltite derivatives and their thermoelectric properties. Chim Techno Acta. 2020;7:26–33. doi:10.15826/chimtech.2020.7.1.04
Klyndyuk AI, Zhuravleva YY, Gundilovich NN. Crystal structure, thermal and electrotransport properties of NdBa1−xSrxFeCo0.5Cu0.5O5+δ (0.02≤x≤0.20) solid solutions. Chimica Techno Acta. 2021;8:20218301. doi:10.15826/chimtech.2021.8.3.01
Huang XY, Wang SY, Rtimi S, Devakumar B. KCa2Mg2V3O12: A novel efficient rare-earth-free self-activated yellow emitting phosphor. J Photochem Photobiol A Chem. 2020;401:112765. doi:10.1016/j.jphotochem.2020.112765
Tsirlin AA, Dikarev EV, Velikodny YA, Shpanchenko RV, Antipov EV. Pb2.63Cd2V3O12, a cation-deficient garnet-type vanadate. Acta Crystallogr C Struct Chem. 2007;63:140–142. doi:10.1107/S0108270107021233
Dutta U, Haque A, Seikh MM. Synthesis, structure and magnetic properties of Ti doped La2MnNiO6 double perovskite. Chim Techno Acta. 2019;6:80–92. doi:10.15826/chimtech.2019.6.3.01
Anokhina IA, Animitsa IE, Buzina AF, Voronin VI, Vykhodets VB, Kurennykh TЕ, Zaikov YP. Synthesis, structure and electrical properties of Li+-doped pyrochlore Gd2Zr2O7. Chim Techno Acta. 2020;7:51–60. doi:10.15826/chimtech.2020.7.2.02
Park JY, Chung JW, Yang HK. Synthesis and photolumines-cence properties of yellow-emitting Ca5(Zn1−xMgx)4(VO4)6 self-activated phosphors. Optik. 2018;155:384–389. doi:10.1016/j.ijleo.2017.11.046
Tolkacheva AS, Shkerin SN, Zemlyanoi KG, Reznitskikh OG, Pershina SV, Khavlyuk PD. Thermal and electrical properties of Ca5Mg4−xZnx(VO4)6 (0≤x≤4). J Therm Anal Calorim. 2019;136:1003–1009. doi:10.1007/s10973-018-7780-z
Lide DR. Handbook of Chemistry and Physics. 84th ed., Boca Raton, FL: CRC Press LLC; 2003. p. 4–93.
Powder Diffraction File PDF4+ ICDD 2018.
Petříček V, Dušek M, Palatinus L, Crystallographic computing system JANA2006: General features. Z. Kristallogr. 2014;229:345–352. doi:10.1515/zkri-2014-1737
Krasnenko TI, Zubkov VG, Tyutyunnik AP, Zolotukhina LV, Vasyutinskaya EF. Crystal structure of β′-Zn2V2O7. Crystallogr. Rep. 2003;48:35–38. doi:10.1134/1.1541739
Miyauchi A, Okabe TH. Production of metallic vanadium by preform reduction process. Mater Trans. 2010;51(6):1102–1108. doi:10.2320/matertrans.M2010027
Shannon RD, Prewitt CT. Effective ionic radii in oxides and fluorides. Acta Crystallogr B Struct Sci Cryst Eng Mater. 1969;25:925–946. doi:10.1107/S0567740869003220
Fotiev AA. Vanadaty: Sostav, Sintez, Struktura, Svoĭstva [Vanadates: composition, synthesis, structure, properties] Moscow: Nauka; 1988. 272 p. Russian.
Rousseau DL, Bauman RP, Porto SPS. Normal mode determination in crystals. J Raman Spectrosc. 1981;10:253–290. doi:10.1002/jrs.1250100152
Koningstein JA, Mortensen OS. Laser-excited phonon Raman spectrum of garnets. J Mol Spectrosc. 1968;27:343-350. doi:10.1016/0022-2852(68)90043-X
Mingsheng P, Mao HK, Dien L, Chao ECT. Raman spectroscopy of garnet-group minerals. Chin J Geochem. 1994;13:176–183. doi:10.1007/BF02838517
Nakamoto K. Infrared and Raman Spectra of Inorganic and Coordination Compounds. Part A: Theory and Applications in Inorganic Chemistry. 6th ed. Hoboken, NJ: John Wiley & Sons, Inc.; 2009. 419 p.
Kolesov BA, Geiger CA. Raman spectra of silicate garnets. Phys Chem Minerals. 1998;25:142–151. doi:10.1007/s002690050097
Moore RK, White WB, Long TV. Vibrational spectra of the common silicates: I. The garnets. Am. Mineral. 1971;56:54–71.
DOI: https://doi.org/10.15826/chimtech.2022.9.2.01
Copyright (c) 2021 Anna S. Tolkacheva, Sergey N. Shkerin, Sofya A. Petrova, Olga M. Fedorova, Svetlana G. Titova, Ivan I. Leonidov

This work is licensed under a Creative Commons Attribution 4.0 International License.
Chimica Techno Acta, 2014–2025
eISSN 2411-1414
Copyright Notice







