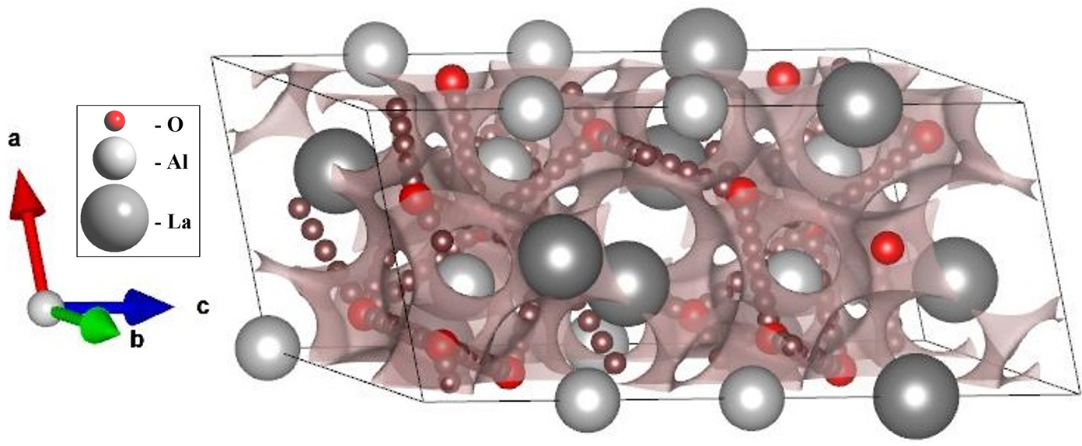
Effect of zinc doping on electrical properties of LaAlO3 perovskite
Abstract
Keywords
Full Text:
PDFReferences
Jacobson AJ. Materials for Solid Oxide Fuel Cells. Chem. Mater. 2010;22(3):660–74. doi:10.1021/cm902640j
Medvedev D, Murashkina A, Pikalova E, Demin A, Podias A, Tsiakaras P. BaCeO3: Materials development, properties and application. Progress in materials science. 2014;60:72–129. doi:10.1016/j.pmatsci.2013.08.001
Sazinas R, Bernuy-Lopez C, Einarsrud M-A. Grande T. Effect of CO2 exposure on the chemical stability and mechanical properties of BaZrO3-ceramics. Journal of the American Ceramic Society. 2016;99:3685–95. doi:10.1111/jace.14395
Gopalan S, Virkar AV. Thermodynamic stabilities of SrCeO3 and BaCeO3 using a molten salt method and galvanic cells. J Electrochem Soc. 1993;140:1060–5. doi:10.1149/1.2056197
Medvedev D, Lyagaeva J, Plaksin S, Demin A, Tsiakaras P. Sulfur and carbon tolerance of BaCeO3 – BaZrO3 proton-conducting materials. Journal of Power Sources. 2015;273:716–23. doi:10.1016/j.jpowsour.2014.09.116
Zitouni H, Tahiri N, Bounagui O El, Ez-Zahraouy H. Electronic, transport and optical properties in perovskite compound LaGaO3. Mater Res Express. 2020;7(3):035501. doi:10.1088/2053-1591/ab778c
Fung K-Z, Chen T-Y. Comparison of dissolution behavior and ionic conduction between Sr and/or Mg doped LaGaO3 and LaAlO3. J. Power Sources. 2004;132(1–2): 1–10. doi:10.1016/j.jpowsour.2003.12.062
Lybye D, Poulsen FW, Mogensen M. Conductivity of A- and B-site doped LaAlO3, LaGaO3, LaScO3 and LaInO3 perovskites. Solid State Ionics. 2000;128:91–103. doi:10.1016/S0167-2738(99)00337-9
Fung K-Z, Chen T-Y. A and B-site substitution of the solid electrolyte LaGaO3 and LaAlO3 with the alkaline-earth oxides MgO and SrO. Journal of Alloys and Compounds. 2004;368(1–2):106–115 doi:10.1016/j.jallcom.2003.08.059
Fung K-Z, Chen T-Y. Cathode-supported SOFC using a highly conductive lanthanum aluminate-based electrolyte. Solid State Ionics. 2011;188(1):64–8. doi:10.1016/j.ssi.2010.09.035
Fabián M, Arias-Serrano BI, Yaremchenko AA, Kolev H, Kaňuchová M, Briančin J. Ionic and electronic transport in calcium-substituted LaAlO3 perovskites prepared via mechanochemical route. Journal of the European Ceramic Society. 2019;39(16):5298–308. doi:10.1016/j.jeurceramsoc.2019.07.038
Park JY, Choi GM. Electrical conductivity of Sr and Mg doped LaAlO3. Solid State Ionics. 2002;154–155:535–40. doi:10.1016/S0167-2738(02)00510-6
Park JY, Choi GM. The effect of Ti addition on the electrical conductivity of Sr- and Mg-doped LaAlO3. Solid State Ionics. 2005;176(37–38):2807–12. doi:10.1016/j.ssi.2005.09.007
Lima E, Villafuerte-Castrejón ME, Saniger JM, Ibarra-Palos A, Sánchez-Sánchez JE, Álvarez LJ. Experimental XRD and NMR, and molecular dynamics study of Sr containing LaAlO3 perovskite. Solid State Ionics. 2008;178(39–40):1944–9. doi:10.1016/j.ssi.2008.01.036
Silva CA, Miranda PEV. Synthesis of LaAlO3 based materials for potential use as methane-fueled solid oxide fuel cell anodes. International journal of hydrogen energy. 2015;40:10002–15. doi:10.1016/j.ijhydene.2015.06.019
Nguyen TL, Dokiya M, Wang S, Tagawa H, Hashimoto T. The effect of oxygen vacancy on the oxide ion mobility in LaAlO3-based oxides, Solid State Ionics. 2000;130:229–41. doi:10.1016/S0167-2738(00)00640-8
Demchuk VA, Shchekina GB, Kostyukov NS. Effect of mineralizing additives ZnO and B2O3 on the sintering temperature of steatite ceramics, Russian Journal of Promising materials. 2013;6:11–4. https://www.elibrary.ru/item.asp?id=19079156&
Sukhanov MV, Pet’kov VI, Firsov DB. Sintering mechanism of high density NZP-ceramics. Inorganic materials. 2011;47(6):674–8. doi:10.1134/S0020168511060197
Sizova AS, Popova NA, Lukin ES. Influence of temperature of synthesis on the properties of ceramics from magnesium oxide containing zinc oxide as a dependent additives. Advances in chemistry and chemical technology [Internet]. 2017 [cited 2020];31(3):105–7. Russian. Available from: https://cyberleninka.ru/article/n/vliyanie-temperatury-sinteza-prekursorov-karbonatov-na-svoystva-keramiki-iz-oksida-magniya-legiruemoy-oksidom-tsinka/viewer
Medvedev DA, Murashkina AA, Demin AK. Formation of Dense Electrolytes Based on BaCeO3 and BaZrO3 for Application in Solid Oxide Fuel Cells: The Role of Solid State Reactive Sintering. Review Journal of Chemistry. 2015;5(3):193–214. doi:10.1134/S2079978015030024
Bakiz B, Guinneton F, Arab M, Benlhachemi A, Villain S, Satre P, Gavarri J-R. Carbonatation and Decarbonatation Kinetics in the La2O3-La2O2CO3 System under CO2 Gas Flows. Advances in Materials Science and Engineering. 2010;2010:1–6. doi:10.1155/2010/360597
Rodríguez-Carvajal J. An introduction to the program FullProf Version 2001. Laboratoire Léon Brillouin, CEA-CNRS, Saclay, France.
Blatov VA, Ilyushin GD, Blatova OA, Anurova NA, Ivanov-Schits AK, Dem'yanets LN. Analysis of migration paths in fast-ion conductors with Voronoi–Dirichlet partition. Acta Crystallographica Section B. 2006;62(6):1010–8. doi:10.1107/S0108768106039425
Blatov VA, Shevchenko AP, Proserpio DM. Applied Topological Analysis of Crystal Structures with the Program Package ToposPro. Crystal Growth & Design. 2014;14(7):3576–86. doi:10.1021/cg500498k
Blatov VA, Anurova NA. Analysis of ion-migration paths in inorganic frameworks by means of tilings and Voronoi-Dirichlet partition: a comparison. Acta Crystallographica Section B. 2009;65(4):426–34. doi:10.1107/S0108768109019880
Adams S, Rao, RP. High power lithium ion battery materials by computational design. Physica Status Solidi A. Applications and Materials Science. 2011;208(8):1746–53. doi:10.1002/pssa.201001116
Sale M, Avdeev M. 3DBVSMAPPER: a program for automatically generating bond-valence sum landscapes. Journal of applied crystallography. 2012;45(5):1054–6. doi:10.1107/S0021889812032906
Nestler T, Meutzner F, Kabanov AA, Zschornak M, Leisegang T, Meyer DC. Combined Theoretical Approach for Identifying Battery Materials: Al3+ Mobility in Oxides. Chemistry of Materials. 2019;31(3):737–47. doi:10.1021/acs.chemmater.8b03631
Kresse G, Furthmüller J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Physical Review B. 1996;54(16):11169–86. doi:10.1103/PhysRevB.54.11169
Henkelman G, Jónsson H. Improved tangent estimate in the nudged elastic band method for finding minimum energy paths and saddle points. The Journal of Chemical Physics. 2000;113(22):9978–85. doi:10.1063/1.1323224
Perdew JP, Burke K, Ernzerhof M. Generalized Gradient Approximation Made Simple. Physical Review Letters. 1996;77(18):3865–8. doi:10.1103/PhysRevLett.77.3865
Johnson D, Inc. ZView: a Software Program for IES Analysis, Version 2.8. Scribner Associates: Southern Pines, NC; 2002. 200 p.
Alves N, Ferraz WB, Faria LO. Synthesis and investigation of the luminescent properties of carbon doped lanthanum aluminate (LaAlO3) for application in radiation dosimetry. Radiation Measurements. 2014;71:90–4. doi:10.1016/j.radmeas.2014.02.008
Kilner JA, Barrow P, Brook RJ. Electrolytes for the high temperature fuel cell; experimental and theoretical studies of the perovskite LaAlO3. Journal of Power Sources. 1978;3:67–80. doi:10.1016/0378-7753(78)80006-8
Benam MR, Abdoshahi N, Sarmazdeh MM. Ab initio study of the effect of pressure on the structural and electronic properties of cubic LaAlO3 by density function theory using GGA, LDA and PBEsol exchange correlation potentials. Physica B. 2014;446:32–8. doi:10.1016/j.physb.2014.04.00609
Deren PJ, Lemanski K, Gagor A, Watras A, Maecka M, Zawadzki M. Symmetry of LaAlO3 nanocrystals as a function of crystallite size. Journal of Solid State Chemistry. 2010;183:2095–100. doi:10.1016/j.jssc.2010.07.01
Skinner SJ, Kilner JA. Oxygen ion conductors. Materials Today. 2003;6(3):30–7. doi:10.1016/S1369-7021(03)00332-8
Ranlov J, Bonanos N, Poulsen FW, Mogensen M. Criteria for prediction of high oxide ion conductivity in perovskite oxides. Solid State Phenomena. 2003;39–40:219–22. doi:10.4028/www.scientific.net/SSP.39-40.219
Wakamura K. Ion conduction in proton- and related defect (super) ionic conductors: Mechanical, electronic and structure parameters. Solid State Ionics. 2009;180(26–27):1343–9. doi:10.1016/j.ssi.2009.08.009
DOI: https://doi.org/10.15826/chimtech.2020.8.1.03
Copyright (c) 2020 Anastasia V. Egorova, Ksenia G. Belova, Irina E. Animitsa, Yelizaveta A. Morkhova, Artem A. Kabanov

This work is licensed under a Creative Commons Attribution 4.0 International License.
Chimica Techno Acta, 2014–2025
eISSN 2411-1414
Copyright Notice






