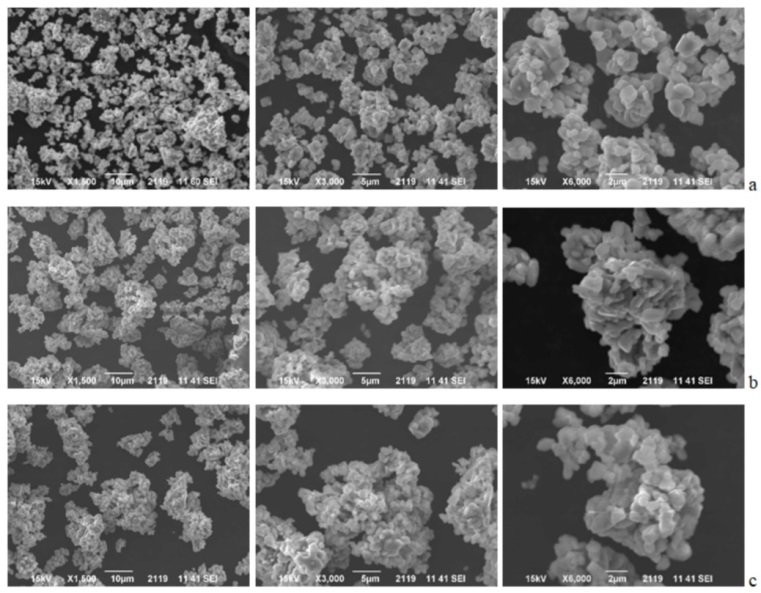
Effect of lithium borate coating on the electrochemical properties of LiCoO2 electrode for lithium-ion batteries
Abstract
Keywords
Full Text:
PDFReferences
Blomgren GE. The development and future of lithium ion batteries. J Electrochem Soc Jpn. 2017;164:A5019–25. doi:10.1149/2.0251701jes
Maximov MYu, Popovich AA, Rumyantsev AM. Influence of passivation coatings synthesized by atomic layer deposition on Li-Ion Batteries cathode cycle life advanced. Materials Research. 2015;1120-1121:730–4. doi:10.4028/www.scientific.net/AMR.1120-1121.730
Fu LJ, Liu H, Li C, Wu YP, Rahm E, Holze R, Wu HQ. Surface modifications of electrode materials for Lithium Ion Batteries. Solid State Sci. 2006;8:113–28. doi:10.1016/j.solidstatesciences.2005.10.019
George SM, Ott AW, Klaus JW. Surface chemistry for atomic layer growth. J Phys Chem. 1996;100:13121–31. doi:10.1021/jp9536763
Zhou A, Wang W, Liu Q, Wang Y, Yao X, Qing F, Li E, Yang T, Zhang L, Li J. Stable, fast and high-energy-density LiCoO2 cathode at high operation voltage enabled by glassy B2O3 modification. J Power Sources. 2017;362:131–9. doi:10.1016/j.jpowsour.2017.06.050
Nagasubramanian A, Yu DYW, Hoster H, Srinivasan M. Enhanced cycling stability of o-LiMnO2 cathode modified by lithium boron oxide coating for lithium-ion batteries. J Solid State Electrochem. 2014;18:1915–22. doi:10.1007/s10008-014-2421-3
Tan SY, Wang L, Bian L, Xu JB, Ren W, Hu PF, Chang AM. Highly enhanced low temperature discharge capacity of LiNi1/3Co1/3Mn1/3O2 with lithium boron oxide glass modification. J Power Sources. 2015;277:139–46. doi:10.1016/j.jpowsour.2014.11.149
Chen S, Chen L, Li Y, Su Y, Lu Y, Bao L, Wang J, Wang, Wu F. Synergistic effects of stabilizing the surface structure and lowering the interface resistance in improving the low-temperature performances of layered lithium-rich materials. ACS Appl Mater Interfaces. 2017;9:8641–8. doi:10.1021/acsami.6b13995
Ohta S, Komagata S, Seki J, Saeki T, Morishita S, Asaoka T. All-solid-state lithium ion battery using garnet-type oxide and Li3BO3 solid electrolytes fabricated by screen-printing. J Power Sources. 2013;238:53–6. doi:10.1016/j.jpowsour.2013.02.073
Jinilian L, Xianming W, Shang C, Jianben L, Zeqiang H. Enhanced high temperature performance of LiMn2O4 coated with Li3BO3 solid electrolyte. Bull Mater Sci. 2013;36:687–91. doi:10.1007/s12034-013-0513-9
Ferreira E, Lima M, Zanotto E. DSC Method for determining the liquidus temperature of glass-forming systems. J Am Ceram Soc. 2010;93:3757–63. doi:10.1111/j.1551-2916.2010.03976.x
Antolini E. LiCoO2: formation, structure, lithium and oxygen nonstoichiometry, electrochemical behaviour and transport properties. Solid State Ionics. 2004;170:159–71. doi:10.1016/j.ssi.2004.04.003
Mukasyan A, Epstein P, Dinka P. Solution combustion synthesis of nanomaterials. Proc Combust Inst. 2007;31:1789–95. doi:10.1016/j.proci.2006.07.052
Mimani T, Patil K. Solution combustion synthesis of nanoscale oxide and their composites. Mater Phys Mech. 2001;4:134–7.
González-Cortés S, Imbert F. Fundamentals, properties and applications of solid catalysts prepared by solution combustion synthesis (SCS). Appl Catal A: General. 2013;452:117–31. doi:10.1016/j.apcata.2012.11.024
Deganello F, Tyagi A. Solution combustion synthesis, energy and environment: Best parameters for better materials. Prog Cryst Growth Charact Mater. 2018;64:23–61. doi:10.1016/j.pcrysgrow.2018.03.001
Jayasankar K, Pandey A, Mishra B, Das S. Mixed fuel synthesis of Y2O3 nanopowder and their applications as dispersed in ODS steel. Adv Powder Technol. 2015;26:1306–13. doi:10.1016/j.apt.2015.07.003
Zhuravlev V, Pachuev A, Nefedova K, Ermakova L. Solution combustion synthesis of LiNi1/3Co1/3Mn1/3O2 as a cathode material for lithium-ion batteries. Int J Self-Propagating High-Temp Synth. 2018;27:154–61. doi:10.3103/S1061386218030147
Santiago E, Andrade A, Paiva-Santos C, Bulhoẽs L. Structural and electrochemical properties of LiCoO2 prepared by combustion synthesis. Solid State Ionics. 2003;158:91–102. doi:10.1016/S0167-2738(02)00765-8
Kalyani P, Kalaiselvi N, Muniyandi N. A new solution combustion route to synthesize LiCoO2 and LiMnO2. J Power Sources. 2002;111:232–8. doi:10.1016/S0378-7753(02)00307-5
Yoon W, Kim K. Synthesis of LiCoO2 using acrylic acid and its electrochemical properties for Li secondary batteries. J Power Sources. 1999;81–82:517–23. doi:10.1016/S0378-7753(98)00226-2
Rodrigues S, Munichandraiah N, Shukla A. Novel solution-combustion synthesis of LiCoO2 and its characterization as cathode material for lithium-ion cells. J Power Sources. 2001;102:322–5. doi:10.1016/S0378-7753(01)00770-4
Hobosyana M, Kharatyan S, Khachatryan H, Grigoryan N. Combustion synthesis of lithium cobaltate. Int J Self-Propagating High-Temp Synth. 2011;20:107–12. doi:10.3103/s1061386211020075
Zhuravlev V, Shikhovtseva A, Ermakova L, Evshchik E, Sherstobitova E, Novikov D, Bushkova O, Dobrovolsky Y. Solution combustion synthesis of lithium cobalt oxide – cathode material for lithium-ion batteries. Int J Electrochem Sci. 2019;14:2965–83. doi:10.20964/2019.03.79
Khaliullin S, Zhuravlev V, Ermakova L, Buldakova L, Yanchenko M, Porotnikova N. Solution combustion synthesis of ZnO using binary fuel (glycine + citric acid). Int. J. Self - Propagating High - Temp. Synth. 2019;28:226–32. doi:10.3103/S1061386219040058
Han C, Zhu C, Saito G, Akiyama T. Glycine/sucrose-based solution combustion synthesis of high-purity LiMn2O4 with improved yield as cathode materials for lithium-ion batteries. Adv Powder Technol. 2015;26:665–71. doi:10.1016/j.apt.2015.01.019
Aruna S, Rajam K. Mixture of fuels approach for the solution combustion synthesis of Al2O3–ZrO2 nanocomposite. Mater Res Bull. 2004;39:157–67. doi:10.1016/j.materresbull.2003.10.005
Bai J, Liu J, Li C, Li G, Du Q. Mixture of fuels approach for solution combustion synthesis of nanoscale MgAl2O4 powders. Adv Powder Technol. 2011;22:72–6. doi:10.1016/j.apt.2010.03.013
Rodríguez-Carvajal J. Recent advances in magnetic structure determination by neutron powder diffraction. Physica B. 1993;192:55–69. doi:10.1016/0921-4526(93)90108-I
Xuanye Y, Hongge Y, Jihua C, Mao H, Feng X, Zhengfu Z, Hongmei X. Solid state synthesis of ultrafine-LiCoO2 by enhanced thermal decomposition of carbonate precursors followed by double-calcining. Solid State Ionics. 2016;289:159–67. doi:10.1016/j.ssi.2016.03.005
Xie J, Imanishi N, Hirano A, Matsumura M, Takeda Y, Yamamoto O. Kinetics investigation of a preferential (104) plane oriented LiCoO2 thin film prepared by RF magnetron sputtering. Solid State Ionics. 2007;178:1218–24. doi:10.1016/j.ssi.2007.06.007
Choi Y, Pyun S, Bae J, Moon S. Effects of lithium content on the electrochemical lithium intercalation reaction into LiNiO2 and LiCoO2 electrodes. J Power Sources. 1995;56:25–30. doi:10.1016/0378-7753(95)80004-Z
Reimers JN, Dahn JR. Electrochemical and in situ X-ray diffraction studies of lithium intercalation in LixCoO2. J Electrochem Soc. 1992;139:2091–7. doi:10.1149/1.2221184
Antolini E. LiCoO2: formation, structure, lithium and oxygen nonstoichiometry, electrochemical behaviour and transport properties. Solid State Ionics. 2004;170:159–71. doi:10.1016/j.ssi.2004.04.003
Amatucci G, Tarascon J, Klein L. CoO2, the end member of the LixCoO2 solid solution. J Electrochem Soc. 1996;143:1114–23. doi:10.1149/1.1836594
Ohzuku T, Ueda A. Solid-state redox reactions of LiCoO2 (R3m) for 4 volt secondary lithium cells. J Electrochem Soc. 1994;141:2010–5. doi:10.1149/1.2059267
DOI: https://doi.org/10.15826/chimtech.2021.8.1.01
Copyright (c) 2020 Zhuravlev V.D., Nefedova K.V., Evshchik E.Yu., Sherstobitova E.A., Kolmakov V.G., Dobrovolsky Y.A., Porotnikova N.M., Korchun A.V., Shikhovtseva A.V.

This work is licensed under a Creative Commons Attribution 4.0 International License.
Chimica Techno Acta, 2014–2025
eISSN 2411-1414
Copyright Notice







