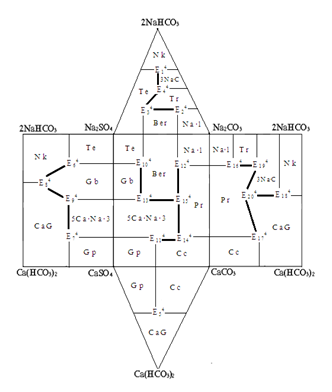
Phase complex of the system Na,Ca||SO4,CO3,HCO3-H2O at 100 ºC
Abstract
The article discusses the results of determining the possible phase equilibria in geometric images of a five-component reciprocal water-salt system of sulfates, carbonates, sodium bicarbonates and calcium at 100 ºC with subsequent construction of its phase complex diagram. The laws that determine the structure of the phase complex diagram of this system are needed to be obtained for the production of scientific data used both as a reference material and also to create the optimal conditions for the recycling of liquid waste industrial production of aluminum-containing sulfate carbonate and bicarbonate salts of sodium and calcium. It was established that the system under study at 100ºC is characterized by the presence of 31 divariant double saturation fields, 25 monovariant trisaturation curves and 14 invariant points.
Keywords
Full Text:
PDFReferences
Spravochnik eksperimental’nykh dannykh po rastvorimosti mnogokomponentnykh vodno-solevykh system [Reference book on experimental data for solubility in multicomponent water-salt systems]. Vol. II., Books. 1–2. Saint-Petersburg: Khimizdat, 2004. 1247 p. Russian.
Soliev L, Jumaev MT. Phase equilibria in the Na,Ca//SO4,CO3,HCO3–H2O system at 0 °C. Chimica Techno Acta. 2019;6(1):24. doi:10.15826/chimtech.2019.6.1.03
Soliev L, Jumaev MT. Phase equilibrium of Na,Ca||SO4,CO3,HCO3-H2O systems at 50 °С. Applied Solid State Chemistry. 2018;4(5):192-8. doi:10.18572/2619-0141-2018-4-5-192-198
Soliev L. [Prediction of structure of multicomponent water-salt systems phase equilibria diagram by means of translation method]. VINITI № 8990‑B87, 1987. 28 p. Russian.
Goroshenko YaG, Soliev L. [New trends in methodology of physicochemical analysis of complex and multicomponent systems (for 125th anniversary of N.S.Kurnakov)]. Zh. Neorg. Khim. 1987;32(7):1676. Russian.
Goroshchenko YaG. Masstsentricheskiy metod izobrazheniya mnogokomponentnykh system [The Center of Mass Method for Multi-component Systems Imaging]. Kiev: Naukova Dumka, 1982. 264 p. Russian.
Tursunbadalov Sh, Soliev L. Phase Equilibria in the quinery Na,K||SO4,CO3,HCO3–H2O system at 75 °C. J Solution Chem. 2015;44(8):1626-39. doi:10.1007/s10953-015-0368-3
Tursunbadalov Sh, Soliev L. Phas Equilibria in multicomponent water-salt system. J Chem Eng Data. 2016;61(7):2209-20. doi:10.1021/acs.jced.5b00875
Soliev L, Jumaev МТ, Turaev RО, Makhmadov KhR. [Solubility in the system Na2SO4-Na2CO3-NaHCO3-H2O at 50 °С]. Chemical Journal of Kazakhstan. 2017;4(60):29. Russian.
Soliev L, Jumaev MT, Nuri V, Valantino N. Phase Equilibria System Na,Ca||SO4,HCO3-H2O at 25 °С. Bulletin of the Tajik National University (Series of natural sciences). 2012;1(3)85:221.
Soliev L, Jumaev MT, Iqbol G, Nizomov IM. Phase Equilibria in the Na,Ca||CO3,HCO3-H2O system at 25 °С. Reports of the Academy of Sciences of the Republic of Tajikistan. 2012; 55(3):220. Russian.
Usmonov MB. Fazovye ravnovesiya i rastvorimost’ v sisteme Na,Ca||SO4,CO3,F-H2O pri 0 i 25 °С [Phase equilibria and solubility in the Na,Ca||SO4,CO3,F-H2O system at 0 and 25 °С] [dissertation]. Tajik State Pedagogical University; 2015. 126 p. Russian.
Valantena N. Fazovye ravnovesiya i rastvorimost’ v sisteme Na,Ca||SO4,HCO3,F-H2O pri 0 i 25 °С [Phase equilibria and solubility in the Na,Ca||SO4,HCO3,F-H2O system at 0 and 25 °С] [dissertation]. Tajik State Pedagogical University; 2016. 121 p. Russian.
Gulomiqbol G. [Phase equilibria and solubility in the Na,Ca||CO3,HCO3,F-H2O system at 0 and 25 °С] [dissertation]. Tajik State Pedagogical University; 2018. Russian.
Soliev L. [Schematic phase equilibria diagrams for multicomponent systems]. Russ J Inorg Chem. 1988;33(5):1305. Russian.
DOI: https://doi.org/10.15826/chimtech.2020.7.2.04
Copyright (c) 2020 Soliev L., Jumaev M.T.

This work is licensed under a Creative Commons Attribution 4.0 International License.
Chimica Techno Acta, 2014–2025
eISSN 2411-1414
Copyright Notice






