Heat of Fusion of Na3AlF6 Eutectic Mixtures with CaF2 and Al2O3
Abstract
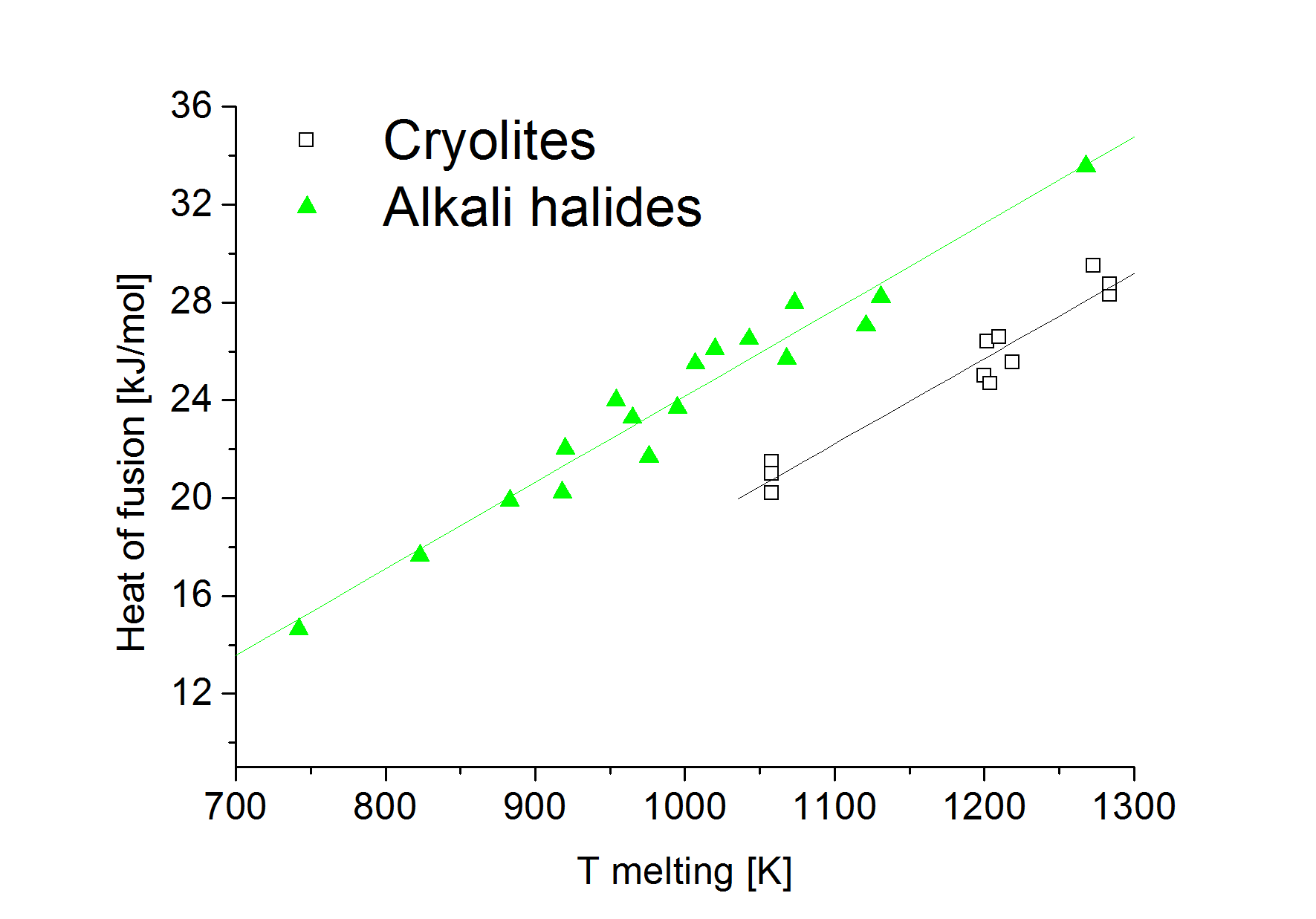
The heat of fusion of eutectic mixtures of sodium cryolite with alumina and calcium fluoride was measured using differential scanning calorimetry. Melting temperatures were found to be in good agreement with literature data. The molar heat of fusion of cryolite salts and eutectic mixtures was found to be directly dependent on melting temperature. The temperature dependence coefficient is the same as that of alkali halides.
Keywords
Full Text:
PDFReferences
Fellner P, Haarberg GM, Híves J, Kvande H, Sterten A. Aluminium Electrolysis. Fundamentals of the Hall-Héroult Process. Aluminium Verlag: Dusseldorf; 2001. 443 p.
Malinovsky M. Cryometric determination of the enthalpy of fusion of sodium cryolite. Chem Zvesty. 1984;38(2):168-72.
Holm B, Gronvold F. Enthalpies of fusion of alkali cryolites determined by drop calorimetry. Acta Chem Scand. 1973;27:2043-50. doi:10.3891/acta.chem.scand.27-2043
O’Brien C, Kelley K. High temperature heat content of cryolite, anhydrous aluminium fluoride and sodium fluoride. J Amer Chem Soc. 1957;79:5616-18. doi:10.1021/ja01578a009
Dolejs D, Baker D. Phase transitions and volumetric properties of cryolite, Na3AlF6: Differential thermal analysis to 100 MPa. Am Mineral. 2006;91:97-103. doi:10.2138/am.2006.1772
Bjorge B, Jenssen B. The calorimetric heat of fusion of Li3AlF6. Acta Chem Scand. 1968;22:1347-48. doi:10.3891/acta.chem.scand.22-1347
Fedotieff PP, Iljinsky WP. Uber die Smellzbarkeit des ternaren Systems:Natriumfluorid, Calciumfluorid, Aluminium Fluorid. Z fur Anorg. und Allgem. Chemie. 1923;129:93-107.
Holm J. The phase diagram of the system Na3AlF6-CaF2 and the constitution of the melt in the system. Acta Chem Scand. 1968;22:1004-12. doi:10.3891/acta.chem.scand.22-1004.
Craig D, Brown J. Phase equilibria in the system CaF2-AlF3-Na3AlF6 and part of the system CaF2-AlF3-Na3AlF6-Al2O3. J Am Ceramic Soc. 1980;63:254-61. doi:10.1111/j.1151-2916.1980.tb10714.x
Tissot P. DTA determination of liquidus temperatures and Al2O3 and AlF3 content in cryolitic melts. Thermochim Acta. 1994;234:245-54. doi:10.1016/0040-6031(94)85147-6
Fenerty A, Hollingshead E. Liquidus curves for aluminum cell electrolyte: III. Systems Cryolite-Alumina with Aluminum Fluoride and Calcium Fluoride. J Electrochem Soc. 1960;107:993-97. doi:10.1149/1.2427588
Gheribi A, Salanne M, Chartrand P. Formulation of Temperature-Dependent Thermal Conductivity of NaF, β-Na3AlF6, Na5Al3F14, and Molten Na3AlF6 Supported by Equilibrium Molecular Dynamics and Density Functional Theory. J Phys Chem C. 2016;120(40):22873-86. doi:10.1021/acs.jpcc.6b07959
Redkin A, Korzun I, Reznitskikh O, Yaroslavtseva T, Zaikov Yu, Kumkov S. Heat of fusion of halide salts and their eutectics. J Therm Anal Calorim. 2018;131:2021-26. doi:10.1007/s10973-017-6650-4
Badenhorst H, Bohmer T. Enthalpy of fusion prediction for the economic optimisation of salt based latent heat thermal energy stores. Journal of Energy Storage. 2018;20:459-72. doi:10.1016/j.est.2018.10.020
Wisniak J. Frederick Thomas Trouton: The man, the Rule and the Ratio. J Chem Educator. 2001;6:55-61. doi:10.1007/s00897000448a
Sawamura H. The relation between entropy of fusion or heat of fusion of metallic elements and their crystal structure. Trans JIM. 1972;13:225-30. doi:10.2320/matertrans1960.13.225
Kaptay G. On the solid/liquid interfacial energies of metals and alloys. J. Mat.Sci. 2018;53: 3767-84. doi:10.1007/s10853-017-1778-y
Redkin A, Zaikov Y, Korzun I, Reznitskikh O, Yaroslavtseva T, Kumkov S. Heat capacity of molten halides. J Phys Chem B. 2015;119:509-12. doi:10.1021/jp509932e
DOI: https://doi.org/10.15826/chimtech.2019.6.3.03
Copyright (c) 2019 Alexander Redkin, Svetlana Pershina, Evgenia Il'ina, Alexander Kataev, Yurii Zaikov

This work is licensed under a Creative Commons Attribution 4.0 International License.
Chimica Techno Acta, 2014–2025
eISSN 2411-1414
Copyright Notice






