Thermodynamic assessment of oxide system In2O3-SnO2-ZnO
Abstract
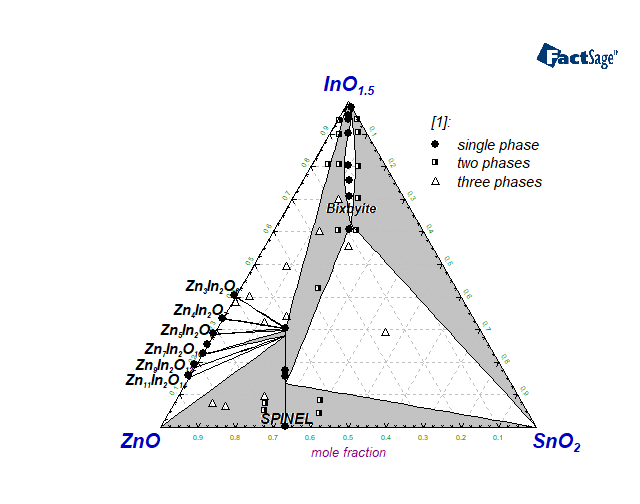
The In2O3-SnO2-ZnO system is of special interest for applications as transparent conducting oxides and also transparent semiconductors. In the present work, a thermodynamic assessment for this system is discussed using all available experimental data on phase equilibria and thermodynamic properties. All sub-systems including elemental combinations were considered in order to generate a self-consistent Gibbs energy dataset for further calculation and prediction of thermodynamic properties of the system. The modified associate species model was used for the description of the liquid phase. Particular attention was given to two significant solid solution phases: Spinel with the formula Zn(2-x)Sn(1-x)In2xO4 based on Zn2SnO4 and Bixbyite based on In2O3 and extending strongly toward the SnZnO3 composition according to the formula In(2‑2x)SnxZnxO3. In addition to the component oxides, nine quasi-binary compounds located in the In2O3-ZnO binary subsystem have also been included in the database as stoichiometric phases.
Keywords
Full Text:
PDFReferences
Harvey SP, Poeppelmeier KR, Mason TO. Subsolidus Phase Relationships in the ZnO–In2O3–SnO2 System. Journal of the American Ceramic Society. 2008;91(11):3683-9. doi:10.1111/j1551-2916.2008.02686.x
Hoel CA, Mason TO, Gaillard J-F, Poeppelmeier KR. Transparent Conducting Oxides in the ZnO-In2O3-SnO2 System. Chemistry of Materials. 2010;22(12):3569-79. doi:10.1021/cm1004592
Granqvist CG, Hultåker A. Transparent and conducting ITO films: new developments and applications. Thin Solid Films. 2002;411(1):1-5. doi:10.1016/S0040-6090(02)00163-3
Palmer GB, Poeppelmeier KR. Phase relations, transparency and conductivity in Ga2O3-SnO2-ZnO. Solid State Sciences. 2002;4(3):317-22. doi:10.1016/S1293-2558(01)01258-4
Minami T, Kakumu T, Takata S. Preparation of transparent and conductive In2O3–ZnO films by radio frequency magnetron sputtering. J Vac Sci Technol. 1996;A14:1704-8. doi:10.1116/1.580323
Moriga T, Edwards DD, Mason TO, Palmer GB, Poeppelmeier KR, Schindler JL, et al. Phase Relationships and Physical Properties of Homologous Compounds in the Zinc Oxide-Indium Oxide System. Journal of the American Ceramic Society. 1998;81(5):1310-6. doi:10.1111/j.1151-2916.1998.tb02483.x
Besmann TM, Spear KE. Thermodynamic modelling of oxide glasses. J Am Ceram Soc. 2002;85(12):2887-94. doi:10.1111/j.1151-2916.2002.tb00552.x
Yazhenskikh E, Jantzen T, Hack K, Müller M. Critical thermodynamic evaluation of oxide system relevant to fuel ashes and slags: Potassium oxide-magnesium oxide-silica. Calphad. 2014;47:35-49. doi:10.1016/j.calphad.2014.05.006
Jantzen T, Hack K, Yazhenskikh E, Müller M. Evaluation of thermodynamic data and phase equilibria in the system Ca-Cr-Cu-Fe-Mg-Mn-S Part II: Ternary and quasi-ternary subsystems. Calphad. 2017;56:286-302. doi:10.1016/j.calphad.2017.01.007
Jantzen T, Hack K, Yazhenskikh E, Müller M. Addition of TiO2 and Ti2O3 to the Al2O3-FeO-Fe2O3-MgO system. Calphad. 2018;62:187-200. doi:10.1016/j.calphad.2018.05.009
SGTE unary database 2017
SGPS - SGTE pure substances database (v13.1) 2017
SGTE Solution database 2017
Pearson WB. A Handbook of Lattice Spacings and Structures of Metals and Alloys. Oxford: Pergamon Press; 1967
Hillert M, Staffansson L-I. Regular Solution Model for Stoichiometric Phases and Ionic Melts. Acta Chem Scand. 1970;24(10):3618-26. doi:10.3891/acta.chem.scand.24-3618
Sundman B, Aagren J. A regular solution model for phases with several components and sublattices, suitable for computer applications. J Phys Chem Solids. 1981;42(Copyright (C) 2018 American Chemical Society (ACS). All Rights Reserved.):297-301. doi:10.1016/0022-3697(81)90144-x
Massalski TB. Binary Alloy Phase Diagrams. Second ed. Metals Park, OH ASM International; 1990
González GB, Mason TO, Okasinski JS, Buslaps T, Honkimäki V. Determination of the Solubility of Tin in Indium Oxide Using In Situ and Ex Situ X-Ray Diffraction. J Am Ceram Soc. 2011:1-7. doi:10.1111/j.1551-2916.2011.04999.x
Ohya Y, Ito T, Kaneko M, Takahashi Y. Solid solubility of SnO2 in In2O3. J Ceram Soc Jpn. 2000;108(9):803-6. doi:10.2109/jcersj.108.1261_803
Enoki H, Echigoya J, Suto H. The intermediate compound in the In2O3-SnO2 system. Journal of Materials Science. 1991;26(15):4110-5. doi:10.1007/bf02402954
Heward WJ, Swenson DJ. Phase equilibria in the pseudo-binary In2O3-SnO2 system. J Mater Sci. 2007;42(Copyright (C) 2018 American Chemical Society (ACS). All Rights Reserved.):7135-40. doi:10.1007/s10853-007-1569-y
FactSage: Facility for the Analysis of Chemichal Thermodynamics. Montreal, Canada: CRCT-ThermFact Inc. and GTT-Technologies; 1976-2015; Available from: http://www.factsage.com/
Bale CW, Bélisle E, Chartrand P, Decterov SA, Eriksson G, Gheribi AE, et al. FactSage thermochemical software and databases, 2010–2016. Calphad. 2016;54:35-53. http://dx.doi.org/10.1016/j.calphad.2016.05.002
Schneider SJ. Phase Equilibria in Systems Involving the Rare Earth Oxides. Part III. The Eu2O3-In2O3 System. J Res Nat Bureau of Standards. 1961;65A(5):429-34. doi:10.6028/jres.065A.044
Fitzner K, Jacob KT. Solubility and activity of oxygen in liquid indium and copper-indium alloys. Journal of the Less Common Metals. 1977;52(2):279-91. doi:10.1016/0022-5088(77)90009-1
Otsuka S, Sano T, Kozuka Z. Activities of oxygen in liquid thallium and indium from electrochemical measurements. MTB. 1980;11(2):313-9. doi:10.1007/bf02668417
Otsuka S, Kozuka Z, Chang YA. Oxygen solubility in liquid indium and oxygen diffusivity in liquid indium and tin. MTB. 1984;15(2):329-35. doi:10.1007/bf02667336
Isomäki I, Hämäläinen M, Gierlotka W, Onderka B, Fitzner K. Thermodynamic evaluation of the In–Sn–O system. Journal of Alloys and Compounds. 2006;422(1):173-7. doi:10.1016/j.jallcom.2005.11.083
McPherson DJ, Hansen M. The system Zirconium-Tin. Trans ASM. 1953;45:915-33
Spandau H, Kohlmeyer EJ. Uber Zinnmonoxyd und sein Verhalten bei hohen Temperaturen. Z Anorg Chem. 1947;254:65-82
Cahen S, David N, Fiorani JM, Maı̂tre A, Vilasi M. Thermodynamic modelling of the O–Sn system. Thermochimica Acta. 2003;403(2):275-85. doi:10.1016/S0040-6031(03)00059-5
Moh GH. Tin-containing mineral systems. I. Tin-iron-sulfur-oxygen system and mineral assemblages in ores. Chem Erde. 1974;33(Copyright (C) 2018 American Chemical Society (ACS). All Rights Reserved.):243-75.
Barin I. Thermochemical Data of Pure Substances: VCH Verlagsgeselschaft mbH; 1995
Li-Zi Y, Zhi-Tong S, Chan-Zheng W. A Thermodynamic Study of Tin Oxides by Coulometric Titration. Journal of Solid State Chemistry. 1994;113(2):221-4. doi:10.1006/jssc.1994.1363
Wriedt HA. The O−Zn (Oxygen-Zinc) system. Journal of Phase Equilibria. 1987;8(2):166. doi:10.1007/bf02873202
Searcy AW. High-Temperature Inorganic Chemistry. Progress in Inorganic Chemistry1962. doi:10.1002/9780470166048.ch2
Anthrop DF, Searcy AW. Sublimation and Thermodynamic Properties of Zinc Oxide. J Phys Chem. 1964;68(8):2335-42. doi:10.1021/j100790a052
Kazenas EK, Tsvetkov JV. The evaporation of oxides. Moscow: Nauka; 1997
Gribchenkova NA, Steblevsky AV, Alikhanyan AS. Vaporization thermodynamics of the ZnO–SnO2 system. The Journal of Chemical Thermodynamics. 2014;70:203-6. doi:10.1016/j.jct.2013.11.010
Gribchenkova NA, Steblevsky AV, Alikhanyan AS. Vaporization in the Ga2O3−ZnO system by high temperature mass spectrometry. The Journal of Chemical Thermodynamics. 2017;115:1-6. doi:10.1016/j.jct.2017.07.009
Gribchenkova NA, Alikhanyan AS, editors. Mass spectrometric investigation of phase equilibria and thermodynamics of ZnO-MxOy (MxOy = Ga2O3, In2O3, SnO2) quasi-binary oxide systems. Knudsen Effusion Mass Spectrometry, KEMS Workshop; 2017 October, 23-25; Jülich, Germany: Forschungszentrum Jülich
Bates JL, Griffin CW, Marchant DD, Garnier JE. Electrical conductivity, Seebeck coefficient, and structure of indium(III) oxide-tin(IV) oxide. Am Ceram Soc Bull. 1986;65:673-8
Kasper H. Neuartige Phasen mit wurtzitähnlichen Strukturen im System ZnO-In2O3. Zeitschrift für anorganische und allgemeine Chemie. 1967;349(3-4):113-23. doi:10.1002/zaac.19673490302
Cannard PJ, Tilley RJD. New intergrowth phases in the ZnO-In2O3 system. Journal of Solid State Chemistry. 1988;73(2):418-26. doi:10.1016/0022-4596(88)90127-2
Nakamura M, Kimizuka N, Mohri T. The phase relations in the In2O3-Fe2ZnO4-ZnO system at 1350°C. Journal of Solid State Chemistry. 1990;86(1):16-40. doi:10.1016/0022-4596(90)90110-J
Kimizuka N, Isobe M, Nakamura M. Syntheses and Single-Crystal Data of Homologous Compounds, In2O3(ZnO)m (m = 3, 4, and 5), InGaO3(ZnO)3, and Ga2O3(ZnO)m (m = 7, 8, 9, and 16) in the In2O3-ZnGa2O4-ZnO System. Journal of Solid State Chemistry. 1995;116(1):170-8. doi:10.1006/jssc.1995.1198
Nakamura M, Kimizuka N, Mohri T, Isobe M. The Phase Relations in the In2O3-Al2ZnO4-ZnO System at 1350°C. Journal of Solid State Chemistry. 1993;105(2):535-49. doi:10.1006/jssc.1993.1246
Park D-H, Son K-Y, Lee J-H, Kim J-J, Lee J-S. Effect of ZnO addition in In2O3 ceramics: defect chemistry and sintering behavior. Solid State Ionics. 2004;172(1):431-4. doi:10.1016/j.ssi.2004.03.029
Enoki H. Oxide transparent electrode materials. Materia. 1995;34(3):344-51. doi:10.2320/materia.34.344
Hansson R, Hayes PC, Jak E. Experimental study of phase equilibria in the Fe-Sn-Zn-O system in air. Can Metall Q. 2004;43(4):545-54. doi:10.1179/cmq.2004.43.4.545
Yu-Sheng S, Tian-Shu Z. Preparation, structure and gas-sensing properties of ultramicro ZnSnO3 powder. Sensors and Actuators B: Chemical. 1993;12(1):5-9. doi:10.1016/0925-4005(93)85003-S
Inagaki M, Kuroishi T, Yamashita Y, Urata M. Syntheses of MSn(OH)6 by coprecipitation and of MSnO3 by thermal decomposition (M = Mg, Co, Zn, Mn, Cd, Ca, Sr, Ba). Zeitschrift für anorganische und allgemeine Chemie. 1985;527(8):193-202. doi:10.1002/zaac.19855270822
Kovacheva D, Petrov K. Preparation of crystalline ZnSnO3 from Li2SnO3 by low-temperature ion exchange. Solid State Ionics. 1998;109(3):327-32. doi:10.1016/S0167-2738(97)00507-9
Mihaiu S, Atkinson I, Mocioiu O, Toader A, Tenea E, Zaharescu M. Phase formation mechanism in the ZnO-SnO2 binary system. Rev Roum Chim. 2011;56(5):465-72
Palmer GB, Poeppelmeier KR, Mason TO. Conductivity and Transparency of ZnO/SnO2-Cosubstituted In2O3. Chem Mater. 1997;9(Copyright (C) 2018 American Chemical Society (ACS). All Rights Reserved.):3121-6. doi:10.1021/cm9704037
Kammler DR, Edwards DD, Ingram BJ, Mason TO, Palmer GB, Ambrosini A, et al. Novel Compound and Solid-Solution Transparent Conducting Oxides for Photovoltaics. In: V.K. Kapur RDM, D. Carlson, G.P. Ceasar, A. Rohatgi, , editor. Electrochem Soc 195th Meeting: Photovoltaics for the 21st Century; Seattle, Washington. Pennington, New Jersey: The Electrochemical Society, Inc., ; 1999. p. 68-77
DOI: https://doi.org/10.15826/chimtech.2018.5.4.02
Copyright (c) 2018 Jantzen T, Hack K, Yazhenskikh E, Müller M

This work is licensed under a Creative Commons Attribution 4.0 International License.
Chimica Techno Acta, 2014–2025
eISSN 2411-1414
Copyright Notice







