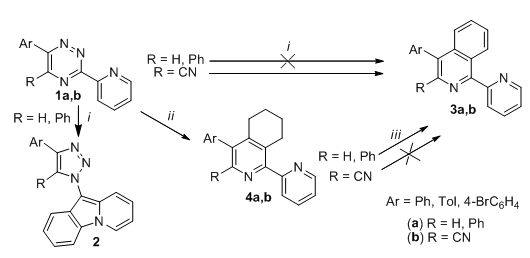
Two mutually complementary synthetic approaches towards 3-substituted 3,4-disubstituted and 1-(2-pyridyl)-substituted isoquinolines
Abstract
Two mutually complementary synthetic approaches towards 3- and 3,4-disubstituted 1-(2-pyridyl) isoquinolines were studied. The aryne-based method was successfully used for the obtaining of the corresponding the 3-cyano-1-(2-pyridyl)isoquinolines in one step/pot reaction, while it is unacceptable for the obtaining of other 1-(2-pyridyl)isoquinolines. The enamine-based approach was successfully applied for the synthesis of other 1-(2-pyridyl)isoquinolines, while it was unacceptable for the obtaining of 3-cyano-1-(2-pyridyl)isoquinolines.
Keywords
Full Text:
PDFReferences
Yoshida S, Hosoya T. The Renaissance and Bright Future of Synthetic Aryne Chemistry. Chem Lett. 2015;44(11):1450-60. doi:10.1246/cl.150839
Wu D, Ge H, Liu SH, Yin J. Arynes in the synthesis of polycyclic aromatic hydrocarbons. RSC Advances. 2013;3(45):22727-38. doi:10.1039/C3RA43804J
Miyabe H. Synthesis of Oxygen Heterocycles via Aromatic C-O Bond Formation Using Arynes. Molecules. 2015;20(7):12558.
Kopchuk DS, Nikonov IL, Khasanov AF, Giri K, Santra S, Kovalev IS, et al. Studies on the interactions of 5-R-3-(2-pyridyl)-1,2,4-triazines with arynes: inverse demand aza-Diels–Alder reaction versus aryne-mediated domino process. Org Biomol Chem. 2018;16(28):5119-35. doi:10.1039/C8OB00847G
Mikata Y, Yamanaka A, Yamashita A, Yano S. Isoquinoline-Based TQEN Family as TPEN-Derived Fluorescent Zinc Sensors. Inorganic Chemistry. 2008;47(16):7295-301. doi:10.1021/ic8002614
Tsuboyama A, Iwawaki H, Furugori M, Mukaide T, Kamatani J, Igawa S, et al. Homoleptic Cyclometalated Iridium Complexes with Highly Efficient Red Phosphorescence and Application to Organic Light-Emitting Diode. J Am Chem Soc. 2003;125(42):12971-9. doi:10.1021/ja034732d
Nikonov IL, Kopchuk DS, Kovalev IS, Zyryanov GV, Khasanov AF, Slepukhin PA, et al. Benzyne-mediated rearrangement of 3-(2-pyridyl)-1,2,4-triazines into 10-(1H-1,2,3-triazol-1-yl)pyrido[1,2-a]indoles. Tetrahedron Lett. 2013;54(48):6427-9. doi:https://doi.org/10.1016/j.tetlet.2013.09.042
Kopchuk DS, Kovalev IS, Khasanov AF, Zyryanov GV, Slepukhin PA, Rusinov VL, et al. A rational protocol for the synthesis of 1-(2-pyridyl)isoquinolines. Mendeleev Commun. 2013;23(3):142-4. doi:10.1016/j.mencom.2013.05.007
Kopchuk DS, Nikonov IL, Zyryanov GV, Kovalev IS, Rusinov VL, Chupakhin ON. Preparation of 3-Cyano-1-(2-Pyridyl)Isoquinolines by Using Aryne Intermediates. Chem Heterocycl Compd. 2014;50(6):907-10. doi:10.1007/s10593-014-1545-9
DOI: https://doi.org/10.15826/chimtech.2018.5.3.04
Copyright (c) 2018 I.L. Nikonov, D.S. Kopchuk, A.F. Khasanov, A.P. Krinochkin, E. S. Starnovskaya, Ya. K. Shtaiz, M.I Savchuk, O.S. Tanya, G.V. Zyryanov, V.L. Rusinov, O.N. Chupakhin

This work is licensed under a Creative Commons Attribution 4.0 International License.
Chimica Techno Acta, 2014–2025
eISSN 2411-1414
Copyright Notice






