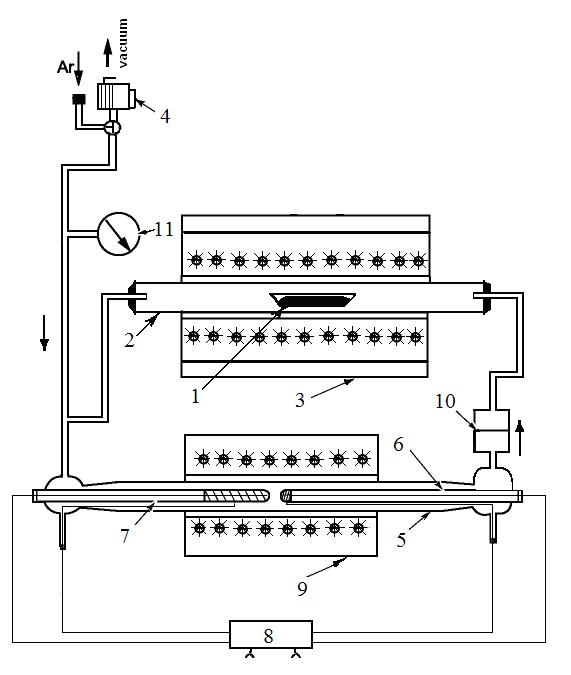
Low-temperature synthesis under low oxygen pressure and thermodynamic properties of YbFe2O4-δ
Abstract
Keywords
Full Text:
PDFReferences
Pyatakov AP, Zvezdin AK. Magnitoelektricheskiye materially I multiferroiki [Magnetoelectric materials and multiferroics]. Uspekhi fizicheskih nauk [Achievements in Physical Sciences]. 2012;182(6):593-620. Russian. doi:10.3367/UFNr.0182.201206b.0593
Kimizuka N, Katsura T. The Standard Free Energy of Formation of YbFe2O4, Yb2Fe3O7, YbFeO3, and Yb3Fe5O12 at 1200 °C. J Solid State Chem. 1975;15:151-7. doi:10.1016/0022-4596(75)90238-8
Kimizuka N, Takenaka A, Sasada Y, Katsura T. A series of new compounds A3+Fe2O4 (A=Ho, Er, Tm, Yb, and Lu). Solid State Commun. 1974;15:1321-3. doi:10.1016/0038-1098(74)91372-6
Katsura T, Sekine T, Kitayama K, Sugihara T, Kimizuka N. Thermodynamic Properties of Fe-Lanthanoid-O Compounds at High Temperatures. J Solid State Chem. 1978;23:43-57. doi.org/10.1016/0022-4596(78)90052-X
Yankin AM, Vedmid’ LB. inventors; Institute of Metallurgy UB RAS, assignee. Isobaric console to synchronous thermoanalyzer. Russian Federation patent RU 88452. 2009 Nov 11. Russian.
Chufarov GI, Men’ AN, Zhuravleva MG, Balakirev VF, Shchepyotkin AA. Termodinamika processov vosstanovleniya okislov metallov [Thermodynamics of metal oxides’ reduction processes]. Moscow: Metallurgy; 1970. 480 p. Russian.
Yankin AM, Balakirev VF, Vedmid’ LB, Fedorova OM. A Static Method for Studying Heterogeneous Equilibria. Russ J Phys Chem. 2003;77(11):1899-1902.
Kato K, Kawada I, Kimizuka N, Katsura T. Die Kristallstruktur von YbFe2O4. Zeitschrift für Kristallographie. 1975;141:314-20. doi:10.1524/zkri.1975.141.3-4.314
Portnoy KI, Timofeeva NI. Kislorodnye soedineniya redkozemelnyh elementov [Oxygen compounds of rare-earth elements]. Moscow: Metallurgy; 1986. 480 p. Russian.
Tret’yakov YuD. Khimiya nestekhiometricheskikh okislov [Chemistry of nonstoichiometric oxides]. Moscow: MGU; 1974. 364 p. Russian.
DOI: https://doi.org/10.15826/chimtech.2018.5.1.03
Copyright (c) 2017 L.B.Vedmid’, V.M. Dimitrov, O.M. Fedorova

This work is licensed under a Creative Commons Attribution 4.0 International License.
Chimica Techno Acta, 2014–2025
eISSN 2411-1414
Copyright Notice






