Potassium carbonate supported efficient synthesis of new diethyl arylphosphoramidates
Abstract
Keywords
Full Text:
PDFReferences
Egron D, Imbach JL, Gosselin G, Aubertin AM, Perigaud C. S-Acyl-2-thioethyl Phosphoramidate Diester Derivatives as Mononucleotide Prodrugs. J Med Chem. 2003;46(21):4564-71. doi:10.1021/jm0308444.
Denmark SE, Chung WJ. Lewis Base Activation of Lewis Acids: Catalytic Enantioselective Glycolate Aldol Reactions. Angew Chem Int Ed. 2008;47(10):1890-2. doi:10.1002/anie.200705499.
Yadav LDS, Srivastava VP, Patel R. The first application of the Baylis–Hillman reaction in azetidine chemistry: a convenient synthesis of azetidine-3-carbonitriles/ carboxylates. Tetrahedron Lett. 2008;49(39):5652-4. doi:10.1016/j.tetlet.2008.07.069.
Minami T, Ogata M, Hirao I, Tanaka M, Agawa T. Synthesis of a 3,1-Benzoxazin-4-one, 2,4(1H,3H)-Quinolinediones, and 2,4(1H,3H)-Quinazolinediones from the Reaction of Phosphoryl-Stabilized Anions Containing no a-Hydrogen Atoms with Isatoic Anhydride. Synthesis. 1982;1982(3):231-3. doi:10.1055/s-1982-29760.
Radkiewicz JL, McAllister MA, Goldstein E, Houk KN. A Theoretical Investigation of Phosphonamidates and Sulfonamides as Protease Transition State Isosteres. J Org Chem. 1998;63(5):1419-28. doi:10.1021/jo971425f.
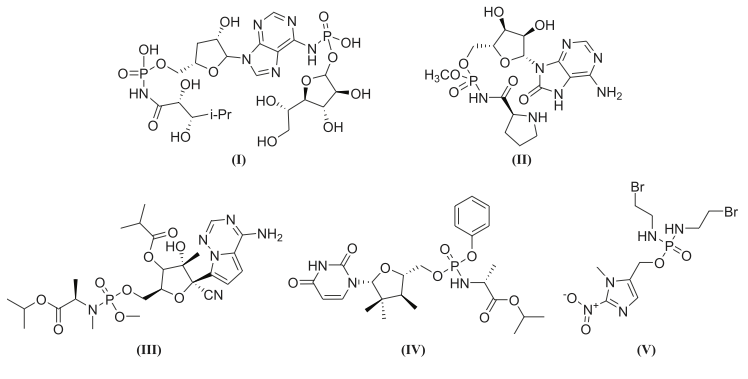
Roberts WP, Tate ME, Kerr A. Agrocin 84 is a 6-N-phosphoramidate of an adenine nucleotide analogue. Nature. 1977;265:379-81. doi:10.1038/265379a0.
Phillips DR, Uramoto M, Isono K, McCloskey JA. Structure of the antifungal nucleotide antibiotic phosmidosine. J Org Chem. 1993;58(4):854-9. doi:10.1021/jo00056a017.
Cho A, Zhang L, Xu J, Lee R, Butler T, Metobo S, Aktoudianakis V, Lew W, Ye H, Clarke M, Doerffler E, Byun D, Wang T, Babusis D, Carey AC, German P, Sauer D, Zhong W, Rossi S, Fenaux M, Mc Hutchison JG, Perry J, Feng J, Ray AS, Kim CU. Discovery of the First C-Nucleoside HCV Polymerase Inhibitor (GS-6620) with Demonstrated Antiviral Response in HCV Infected Patients. J Med Chem. 2014;57:1812-25. doi:10.1021/jm400201a.
Charlton M, Gane E, Manns MP, Brown RS, Curry MP, Kwo PY, Fontana RJ, Gilroy R, Teperman L, Muir AJ, McHutchison JG, Symonds WT, Brainard D, Kirby B, Dvory-Sobol H, Denning J, Arterburn S, Samuel D, Forns X, Terrault NA. Sofosbuvir and Ribavirin for Treatment of Compensated Recurrent Hepatitis C Virus Infection After Liver Transplantation. Gastroenterology. 2015;148(1):108-17. doi:10.1053/j.gastro.2014.10.001.
World Health Organization. Proposed INN: List 111. WHO Drug Information [Internet]. 2014[cited 2017 Jun 14];28(2):211-94. Available from: http://www.who.int/medicines/publications/druginformation/PL_111.pdf.
Adelfinskaya O, Herdewijn P. Amino Acid Phosphoramidate Nucleotides as Alternative Substrates for HIV-1 Reverse Transcriptase. Angew Chem Int Ed. 2007;46(23):4356-8. doi:10.1002/anie.200605016.
Gao X, Tang Z, Lu M, Liu H, Jiang Y, Zhao Y, Cai Z. Suppression of matrix ions by N-phosphorylation labeling using matrix-assisted laser desorption–ionization time-of-flight mass spectrometry. Chem Commun. 2012;48(82):10198-200. doi:10.1039/c2cc36091h.
Overtveldt MV, Heugebaeurt TSA, Verstraeten I, Geelen D, Stevens CV. Phosphonamide pyrabactin analogues as abscisic acid agonists. Org Biomol Chem. 2015;13(18):5260-4. doi:10.1039/C5OB00137D.
Bakibaev AA, Yagovkin AY, Vostretsov SN. Methods of synthesis of nitrogen-containing heterocycles using ureas and related compounds. Russ Chem Rev. 1998;67(4): 295-314. doi:10.1070/RC1998v067n04ABEH000295.
Milen M, Abranyi-Balogh P, Balogh G, Drahos L, Keglevich G. A Study on the Phosphorylation of Indole, Imidazole, Carbazole, and Phenothiazine Derivatives. Phosphorus, Sulfur Silicon Relat Elem. 2012;187(9):1091-100. doi:10.1080/10426507.2012.668986.
Davis TA, Wilt JC, Johnston JN. Bifunctional Asymmetric Catalysis: Amplification of Brønsted Basicity Can Orthogonally Increase the Reactivity of a Chiral Brønsted Acid. J Am Chem Soc. 2010;132(9):2880-2. doi:10.1021/ja908814h.
Xiang DF, Bigley AN, Ren Z, Xue H, Hull KG, Romo D, Raushel FM. Interrogation of the Substrate Profile and Catalytic Properties of the Phosphotriesterase from Sphingobium sp. Strain TCM1: An Enzyme Capable of Hydrolyzing Organophosphate Flame Retardants and Plasticizers. Biochemistry. 2015;54(51):7539-49. doi:0.1021/acs.biochem.5b01144.
DOI: https://doi.org/10.15826/chimtech/2017.4.2.030
Copyright (c) 2017 P. V. Ramana, B. S. Krishna, N. B. Reddy, G. Sravaya, G. V. Zyryanov, C. S. Reddy

This work is licensed under a Creative Commons Attribution 4.0 International License.
Chimica Techno Acta, 2014–2025
eISSN 2411-1414
Copyright Notice






