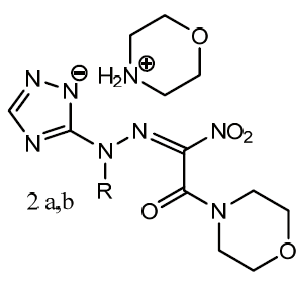
The features of nucleophilic substitution of the nitro group in 4-alkyl-6-nitro-1,2,4-triazolo[5,1-c][1,2,4]triazines
Abstract
Keywords
Full Text:
PDFReferences
Reddy PL, Khan SI, Ponnan P, Tripathi M, Rawat DS. Design, synthesis and evaluation of 4-aminoquinoline-purine hybrids as potential antiplasmodial agents. Eur J Med Chem. 2017;126:675–86. doi:10.1016/j.ejmech.2016.11.057
Charushin VN, Chupakhin ON, editors. Metal Free C-H Functionalisation of Aromatics. Nucleophilic Displacement of Hydrogen. Cham (Switzerland): Springer International Publishing; c2014. 283 p. doi:10.1007/978-3-319-07019-3
Ding H, Chen Zh, Zhang C, Xin T, Wang Y, Song H, Jiang Y, Chen Yu, Xu Yo, Tan Ch. Synthesis and Cytotoxic Activity of Some Novel N-Pyridinyl-2-(6-phenylimidazo[2,1-b]thiazol-3-yl)acetamide Derivatives. Molecules. 2012;17(4):4703–16. doi:10.3390/molecules17044703
Tewari S, Chauhan B, Fatima NCh. Syntheses and antifi larial profi le of 7-chloro-4-(substituted amino) quinolines: a new class of antifilarial agents. Bioorg Med Chem Lett. 2000;10(13):1409–12. PMID: 10888320
Van der Plas HC. Advances in Heterocyclic Chemistry. Amsterdam (Netherlands): Elsevier Academic Press; 1999. Chapter III, SN(ANRORC) Reactions in Azaheterocycles Containing an “Inside” Leaving Group; p. 87-151. doi:10.1016/S0065-2725(08)60810-7
Uliankina IV, Zavodskaya AV, Parfenov VE, Gidaspov AA, Shiryaev AK, Selezneva EV, Bakharev VV. Alkylation of [1,2,4]triazolo[1,5-a][1,3,5]triazine-7(3H)-ones. Chem Heterocycl Compd. 2016;52(12):1054–60. doi:10.1007/s10593-017-2006-z
Parfenov VE, Bakharev VV, Gidaspov AA, Shiryaev AK, Slepukhin PA. Alkylation reactions of 5-amino-substituted tetrazolo[1,5-a][1,3,5]triazin-7(3H)-ones with alkyl halides. Chem Heterocycl Compd. 2016;52(12):1061–9. doi:10.1007/s10593-017-2007-y
Danagulyan GG, Panosyan GA, Sahakyan LG. Recyclization of pyrimidinium salts into 1,2,4-triazole derivatives. Chem Heterocycl Compd. 2007;43(8):1175–9. doi:10.1007/s10593-007-0155-1
Rusinov VL, Chupakhin ON, Ulomsky EN, Petrov AY, Sharonov EA. Nitroazines. 9. Characteristic features of nucleophilic substitution of the nitro group in dihydroazolo[5,1-c][1,2,4]triazines. Chem Heterocycl Compd. 1989;25(2):209-13. doi:10.1007/BF00479921
Rusinov VL, Chupakhin ON, Ulomski EN, Rusinov GL, Chernyshev AI, Aleksandrov GG. Nitroazines. 7. Alkylation of 6-nitro-7-oxo-4,7-dihydroazolo[5,1-c][1,2,4]-triazines and identification of the products. Chem Heterocycl Compd. 1987:23(11):1543–50. doi:10.1007/BF00479378
DOI: https://doi.org/10.15826/chimtech.2017.4.1.020
Copyright (c) 2017 E. N. Ulomsky, D. N. Lyapustin, V. V. Fedotov, O. S. Eltsov, I. M. Sapozhnikova, D. N. Kozhevnikov, E. M. Mukhin

This work is licensed under a Creative Commons Attribution 4.0 International License.
Chimica Techno Acta, 2014–2025
eISSN 2411-1414
Copyright Notice






