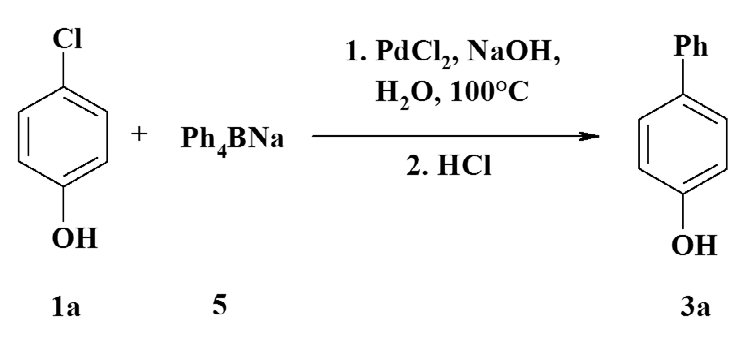
Chlorophenols in organic synthesis
Abstract
Keywords
Full Text:
PDFReferences
McElroy WT, DeShong P. Synthesis of the CD-ring of the anticancer agent streptonigrin: studies of aryl-aryl coupling methodologies. Tetrahedron. 2006;62(29):6945–54. doi:10.1016/j.tet.2006.04.074
Torres JC, Pinto AC, Garden SJ. Application of a catalytic palladium biaryl synthesis reaction, via C–H functionalization, to the total synthesis of Amaryllidaceae alkaloids. Tetrahedron. 2004;60(44)9889–900. doi:10.1016/j.tet.2004.08.030
Miyaura N, Yanagi T, Suzuki A. The Palladium-Catalyzed Cross-Coupling Reaction of Phenylboronic Acid with Haloarenes in the Presence of Bases. Synth Commun. 1981;11(7):513-9. doi:10.1080/00397918108063618
Yuan B, Pan Y, Li Y, Yin B, Jiang H. A Highly Active Heterogeneous Palladium Catalyst for the Suzuki–Miyaura and Ullmann Coupling Reactions of Aryl Chlorides in Aqueous Media. Angew Chem Int Ed. 2010;49(24):4054-8. doi:10.1002/anie.201000576
Astruc D, Lu F, Aranzaes JR. Nanoparticles as recyclable catalysts: The frontier between homogeneous and heterogeneous catalysis. Angew Chem Int Ed. 2005;44(48):7852-72. doi:10.1002/anie.200500766
Gallon BJ, Kojima RW, Kaner RB, Diaconescu PL. Palladium nanoparticles supported on polyaniline nanofibers as a semi-heterogeneous catalyst in water. Angew Chem Int Ed. 2007;46(38):7251-4. doi:10.1002/anie.200701389
Bumagin NA, Bykov VV. Ligandless palladium catalyzed reactions of arylboronic acids and sodium tetraphenylborate with aryl halides in aqueous media. Tetrahedron. 1997;53(42):14437-50. doi:10.1016/S0040-4020(97)00936-8
Zeng J, Liu KM, Duan XF. Selective Co/Ti Cooperatively Catalyzed Biaryl Couplings of Aryl Halides with Aryl Metal Reagents. Org Lett. 2013;15(20):5342–5. doi:10.1021/ol402599f
Huang J, Nolan SP. Efficient cross-coupling of aryl chlorides with aryl grignard reagents (Kumada reaction) mediated by a palladium/imidazolium chloride system. J Am Chem Soc. 1999;121(42):9889-90. doi:10.1021/ja991703n
Lee DH, Qian Y, Park JH, Lee JS, Shim SE, Jin MJ. A Highly Active and General Catalyst for the Stille Coupling Reaction of Unreactive Aryl, Heteroaryl, and Vinyl Chlorides under Mild Conditions. Adv Synth Catal. 2013;355(9):1729-35. doi:10.1002/adsc.201300075
Dichiarante V, Fagnoni M, Albini A. Metal-free synthesis of sterically crowded biphenyls by direct Ar-H substitution in alkyl benzenes. Angew Chem Int Ed. 2007;46(34):6495-8. doi:10.1002/anie.200701462.
Ellis GP, Romney-Alexander TM. Cyanation of aromatic halides. Chem Rev. 1987;87(4):779-94. doi:10.1021/cr00080a006
Grushin VV, Alper H. Transformations of chloroarenes, catalyzed by transitionmetal complexes. Chem Rev. 1994;94(4):1047-62. doi:10.1021/cr00028a008
Arvela RK, Leadbeater NE. Rapid, Easy Cyanation of Aryl Bromides and Chlorides Using Nickel Salts in Conjunction with Microwave Promotion. J Org Chem. 2003;68(23):9122-25. doi:10.1021/jo0350561
Franck HG, Stadelhofer JW. Industrial Aromatic Chemistry. Berlin: Springer-Verlag; c1988. 486 p. doi:10.1007/978-3-642-73432-8
Surburg H, Panten J. Common Fragrance and Flavor Materials. 5th ed. Weinheim (Germany): Wiley-VCH; 2006. 330 p. doi:10.1002/3527608214
Olah GA. Friedel-Crafts Chemistry. New York: Wiley; 1973. 581 p.
Colbon P, Ruan J, Purdie M, Xiao J. Direct acylation of aryl chlorides with aldehydes by palladium-pyrrolidine Co-catalysis. Org Lett. 2010;12(16):3670-3673. doi:10.1021/ol101466g
Semmes JG, Bevans SL, Mullins CH, Shaughnessy KH. Arylation of diethyl malonate and ethyl cyanoacetate catalyzed by palladium/di-tert-butylneopentylphosphine. Tetrahedron Lett. 2015;56(23):3447–3450. doi:10.1016/j.tetlet.2015.01.072
Kozaki M, Okada K. Snowflake-Like Dendrimers via Site-Selective Synthesis of Dendrons. Org Lett. 2004;6(4):485-8. doi:10.1021/ol036011p
Yatabe T, Suzuki Y, Kawanishi YJ. Liquid crystalline conjugated oligomers: Synthesis and mesomorphic properties of laterally and terminally alkyl-substituted oligo(1,4- phenyleneethynylene)s. J Mater Chem. 2008;18(37):4468-77. doi:10.1039/b808036d
Dasaradhan C, Kumar YS, Prabakaran K, Khan FRN, Jeong ED, Chung EH. Efficient and convenient copper-free Pd(OAc)2/Ruphos-catalyzed Sonogashira coupling in the preparation of corfin analogues. Tetrahedron Lett. 2015;56(6):784-8. doi:10.1016/j.tetlet.2014.12.059
Aronica LA, Giannotti L, Giuntini S, Caporusso AM. Synthesis of 2-Alkylideneisochromans by Cyclocarbonylative Sonogashira Reactions. Eur J Org Chem. 2014;31:6858-62. doi:10.1002/ejoc.201402979
Gautam P, Maragani R, Misra R. Tuning the HOMO-LUMO gap of donor-substituted benzothiazoles. Tetrahedron Lett. 2014;55(50):6827-30. doi:10.1016/j.tetlet.2014.10.094
Prabhu RN, Pal S. Copper-free Sonogashira reactions catalyzed by a palladium(II) complex bearing pyrenealdehyde thiosemicarbazonate under ambient conditions. Tetrahedron Lett. 2015;56(37):5252–6. doi:10.1016/j.tetlet.2015.07.076
Egan BA, Burton PM. Synthesis of 3-aryl-1H-indazoles via iridium-catalysed C-H borylation and Suzuki-Miyaura coupling. RSC Adv. 2014;4(53):27726–9. doi:10.1039/c4ra04235b
Brown HC, Kulkarni SV, Racherla US. Chiral synthesis via organoboranes. 39. A facile synthesis of γ-substituted-γ-butyrolactones in exceptionally high enantiomeric purity. J Org Chem. 1994;59(2):365-9. doi:10.1021/jo00081a014
Lambert JD, Rice JE, Hong J, Hou Z, Yang CS. Synthesis and biological activity of the tea catechin metabolites, M4 and M6 and their methoxy-derivatives. Bioorg Med Chem Lett. 2005;15(4):873-6. doi:10.1016/j.bmcl.2004.12.070
Asano M, Inoue M, Katoh T. Model studies towards the total synthesis of GKK1032s, novel antibiotic anti-tumor agents: Enantioselective synthesis of the alkyl aryl ether portion of GKK1032s. Synlett. 2005;17:2599-602. doi:10.1055/s-2005-917112
Protti S, Fagnoni M, Albini A. Benzyl (phenyl) γ- and δ-lactones via photoinduced tandem Ar-C, C-O bond formation. J Am Chem Soc. 2006;128(33):10670-1. doi:10.1021/ja0627287
Cragg GM, Grothaus PG, Newman DJ. Impact of natural products on developing new anti-cancer agents. Chem Rev. 2009;109(7):3012-43. doi:10.1021/cr900019
Singh IP, Bodiwala HS. Recent advances in anti-HIV natural products. Nat Prod Rep. 2010;27(12):1781-800. doi:10.1039/c0np00025f
Cavalli A, Bolognesi M L, Minarini A, Rosini M, Tumiatti V, Recanatini M, Melchiorre C. Multi-target-directed ligands to combat neurodegenerative diseases. J Med Chem. 2008;51(3):347-70. doi:10.1021/jm7009364
Bariwal J, Eycken EV. C-N bond forming cross-coupling reactions: An overview. Chem Soc Rev. 2013;42(24):9283-303. doi:10.1039/c3cs60228a
Reddy LP, Arundhathi R, Rawat DS. Cu(0)@Al2O3/SiO2 NPs: an efficient reusable catalyst for the cross coupling reactions of aryl chlorides with amines and anilines. RSC Adv. 2015;5(112):92121-7. doi:10.1039/C5RA19337K
Anderson KW, Tundel RE, Ikawa T, Altman RA, Buchwald SL. Monodentate phosphines provide highly active catalysts for Pd-catalyzed C-N bond-forming reactions of heteroaromatic halides/amines and (H)N-heterocycles. Angew Chem Int Ed. 2006;45(39):6523–7. doi:10.1002/anie.200601612
Shen Q, Shekhar S, Stambuli JP, Hartwig JF. Highly reactive, general, and long-lived catalysts for coupling heteroaryl and aryl chlorides with primary nitrogen nucleophiles. Angew Chem Int Ed. 2005;44(9):1371–5. doi:10.1002/anie.200462629
Ikawa T, Barder TE, Biscoe MR, Buchwald SL. Pd-catalyzed amidations of aryl chlorides using monodentate biaryl phosphine ligands: A kinetic, computational, and synthetic investigation. J Am Chem Soc. 2007;129(43):13001-7. doi:10.1021/ja0717414
Wang Y, Chackalamannil S, Hu Z, Clader JW, Greenlee W, Billard W, Binch H, Crosby G, Ruperto V, Duffy RA, McQuade R, Lachowicz JE. Design and synthesis of piperidinyl piperidine analogues as potent and selective M2 muscarinic receptor antagonists. Bioorg Med Chem Lett. 2000;10(20):2247–50. doi:10.1016/S0960-894X(00)00457-1
Nielsen SF, Nielsen EO, Olsen GM, Liljefors T, Peters D. Novel potent ligands for the central nicotinic acetylcholine receptor: Synthesis, receptor binding, and 3D-QSAR analysis. J Med Chem. 2000;43(11):2217–26. doi:10.1021/jm990973d
Alcaraz ML, Atkinson S, Cornwall P, Foster AC, Gill DM, Humphries LA, Keegan PS, Kemp R, Merifield E, Nixon RA, Noble AJ, O’Beirne D, Patel ZM, Perkins J, Rowan P, Sadler P, Singleton JT, Tornos J, Watts AJ, Woodland IA. Efficient syntheses of AZD4407 via thioether formation by nucleophilic attack of organometallic species on sulphur. Org Process Res Dev. 200;9(5):555–69. doi:10.1021/op0500483.
Kaldor SW, Kalish VJ, Davies II JF, Shetty BV, Fritz JE, Appelt K, Burgess JA, Campanale KM, Chirgadze NY, Clawson DK, Dressman BA, Hatch SD, Khalil DA, Kosa MB, Lubbehusen PP, Muesing MA, Patick AK, Reich SH, Su KS, Tatlock JH. Viracept (nelfinavir mesylate, AG1343): A potent, orally bioavailable inhibitor of HIV-1 protease. J Med Chem. 1997;40(24):3979–85. doi:10.1021/jm9704098
Martino de G, Edler MC, Regina la G, Coluccia A, Barbera MC, Barrow D, Nicholson RI, Chiosis G, Brancale A, Hamel E, Artico M, Silvestri R. New arylthioindoles: Potent inhibitors of tubulin polymerization. 2. Structure-activity relationships and molecular modeling studies. J Med Chem. 2006;49(3):947–54. doi:10.1021/jm050809s
Martino de G, Regina la G, Coluccia A, Edler MC, Barbera MC, Brancale A, Wilcox E, Hamel E, Artico M, Silvestri R. Arylthioindoles, potent inhibitors of tubulin polymerization. J Med Chem. 2004;47(25):6120–3. doi:10.1021/jm049360d
Fernandez-Rodriguez MA, Shen Q, Hartwig JF. Highly efficient and functional-group-tolerant catalysts for the palladium-catalyzed coupling of aryl chlorides with thiols. Chem - Eur J. 2006;12(30):7782-96. doi:10.1002/chem.200600949
Fernandez-Rodriguez MA, Shen Q, Hartwig JF. A general and long-lived catalyst for the palladium-catalyzed coupling of aryl halides with thiols. J Am Chem Soc. 2006;128(7):2180–1. doi:10.1021/ja0580340
Miyaura N. Cross-Coupling Reactions: A Practical Guide. Berlin: Springer-Verlag; c2002. 248 p. (Topics in Current Chemistry, vol. 219). doi:10.1007/3-540-45313-X
Billingsley KL, Barder TE, Buchwald SL. Palladium-catalyzed borylation of aryl chlorides: Scope, applications, and computational studies. Angew Chem Int Ed. 2007;46(28):5359–63. doi:10.1002/anie.200701551
DOI: https://doi.org/10.15826/chimtech.2016.3.3.013
Copyright (c) 2016 V. V. Mitin, E. A. Ivanova, P. E. Prokhorova, Yu. Yu. Morzherin

This work is licensed under a Creative Commons Attribution 4.0 International License.
Chimica Techno Acta, 2014–2025
eISSN 2411-1414
Copyright Notice






