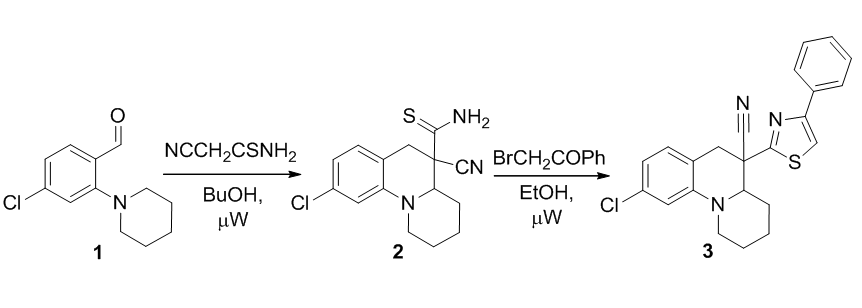
Synthesis of 5-thiocarbomoyl- and 5-(thiazol-2-yl)-2,3,4,4a,5,6-hexahydro-1Н-pyrido[1,2-a]quinoline-5-carbonitriles
Abstract
Keywords
Full Text:
PDFReferences
Meth-Cohn O, Suschitzky H. Heterocycles by Ring Closure of Ortho-Substituted t-Anilines (The t-Amino Effect). Adv Heterocycl Chem. 1972;14:211-78. doi:10.1016/S0065-2725(08)60954-X
Matyus P., Elias O., Tapolcsanyi P., Polonka-Balint A., Halasz-Dajka B. Ring-Closure Reactions of ortho-Vinyl-tert-anilines and (Di)Aza-Heterocyclic Analogues via the tert-Amino Effect: Recent Developments. Synthesis. 2006;16:2625-39. doi:10.1055/s-2006-942490
Meth-Cohn O. The t-Amino Effect: Heterocycles Formed by Ring Closure of ortho-Substituted t-Anilines. Adv Heterocycl Chem. 1996;65:1-37. doi:10.1016/S0065-2725(08)60294-9
Verboom W, Reinhoudt DN. “Tert-amino effect” in heterocyclic synthesis. Ring closure reactions of N,N-dialkyl-1,3-dien-1-amines. Recueil des Travaux Chimiques des Pays-Bas. 1990;109(5):311-24. doi:10.1002/recl.19901090502
Glukharev TV, Klimova EP, Platonova AYu, Morzherin YuYu. Interaction of 2-piperazinobenzaldehyde with cyanoacet(thio)amide: Stereoselective cyclization by the “tert-amino effect” mechanism. Chem Heterocycl Compd. 2008;44(6):759-61. doi:10.1007/s10593-008-0097-2
Li Dzh. Imennyye reaktsii. Mekhanizmy organicheskikh reaktsyy. Moscow: BINOM; 2006. 456 p. Russian.
Deeva EV, Glukhareva TV, Zybina NA, Morzherin YuYu. Stereoselective synthesis of spiro derivatives of 2,4-dimethyl-2,3,4,4a,5,6-hexahydro-6H-benzo[c]quinolizine. Russ Chem Bull. 2005;54(6):1537-8. doi:10.1007/s11172-005-0444-8
Menta E, Da Re G, Grugni M., authors; Cti Europe S.R.L., assignee. Derivatives of chromen-2-one as inhibitors of vegf production in mammalian cells. United States patent US20060122387 A1. 2006 Jun 8.
DOI: https://doi.org/10.15826/chimtech.2015.2.2.010
Copyright (c) 2015 A. A. Poluikova, A. Yu. Platonova, T. V. Glukhareva, Yu. Yu. Morzherin

This work is licensed under a Creative Commons Attribution 4.0 International License.
Chimica Techno Acta, 2014–2025
eISSN 2411-1414
Copyright Notice






